Advertisements
Advertisements
प्रश्न
Which of the following does not belong to the same homologous series?
विकल्प
CH4
C2 H6
C3 H8
C4 H8
उत्तर
C4 H8
Explanation -
The succeeding members of the homologues series differ by a —CH2 - unit. The general formula for the alkane is CnH2n+2. Methane (CH4), ethane (C2H6) and propane (C3H8) are members of homologues series for alkanes while butene (C4H8) is the fourth member of homologues series for alkene.
APPEARS IN
संबंधित प्रश्न
Write the molecular formula of first two members of homologous series having functional group – OH
Fill in the following blank with suitable word:
Ethene and ethyne are examples of ..... hydrocarbons.
The molecular formula of the third member of the homologous series of ketones is:
(a) C4H8O
(b) C3H6O
(c) C5H10O
(d) C6H12O
Succeeding members of a homologous series differ by ______.
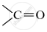 .
.
Define homologous series of organic compounds. List its two characteristics. Write the name and formula of the first member of the series of alkenes.
Two statements are given - one labeled Assertion (A) and the other labeled Reason (R).
Assertion (A): In a homologous series of alcohols, the formula for the second member is C2H5OH and the third member is C3H7OH.
Reason (R): The difference between the molecular masses of the two consecutive members of a homologous series is 144.
Saturated hydrocarbon : Single bond : : Unsaturated hydrocarbon : _______
Consider the carbon compounds having following molecular formula:
(i) C2H2 (ii) C2H5 (iii) C3H7OH (iv) C2H6COOH (v) CH3CHO
- Identify which one of the above compounds, is a member of aldehyde series.
- Write the general formula of the series to which compound C2H2 belongs.
- Which one of the above compounds has triple bonds between carbon-carbon atoms?
- Write the molecular formula of the first member of the homologous series to which the compound C3H7OH belongs.
