Advertisements
Advertisements
प्रश्न
Why is the C-O bond length in phenols less than that in methanol?
उत्तर
The carbon-oxygen bond length in phenol (136 pm) is somewhat shorter than that in methanol. This is owing to the partial double bond character of the unshared electron pair of oxygen conjugated with the aromatic ring and the sp2 hybridised state of carbon to which oxygen is connected
APPEARS IN
संबंधित प्रश्न
Write the structure of the product of the following reaction:
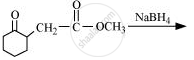
Write the structure of the compound whose IUPAC name is as follows:
1-Phenylpropan-2-ol
Write the structure of the compound whose IUPAC name is as follows:
3, 5-Dimethylhexane−1, 3, 5-triol
Write the structure of the compound whose IUPAC name is as follows:
2, 3-Diethylphenol
Write the structure of the compound whose IUPAC name is as follows:
Cyclohexylmethanol
Write the structure of the compound whose IUPAC name is as follows:
3-Cyclohexylpentan-3-ol
What is the structure and IUPAC name of glycerol?
Which of the following does not form phenol or peroxide?
Diethyl ether finds are in medicine as
Write the structure of the compound whose IUPAC name is as follows:
4-Chloro-3-ethylbutan-1-ol
