Advertisements
Advertisements
प्रश्न
Why is the conversion of ethanol into ethanoic acid an oxidation reaction?
उत्तर
Ethanol is converted into ethanoic acid by taking up oxygen released by potassium permanganate in the reaction mixture. Since this reaction involves adding oxygen to ethanol, it is an oxidation reaction.
The chemical equation for the above reaction is as follows:
\[\ce{CH3CH2OH + 2[O] -> CH3COOH + H2O}\]
संबंधित प्रश्न
When ethanol reacts with ethanoic acid in the presence of conc. H2SO4, a substance with fruity smell is produced. Answer the following:-
(i) State the class of compounds to which the fruity smelling compounds belong. Write the chemical equation for the reaction and write the chemical name of the product formed.
(ii) State the role of conc. H2SO4 in the reaction.
A student adds 2 mL of acetic acid to a test tube containing 2 mL of distilled water. He then shakes the test tube well and leaves it to settle for some time. After about 5 minutes he observes that in the test tube there is :
(A) a clear transparent colourless solution
(B) a clear transparent pink solution
(C) a precipitate settling at the bottom of the test tube
(D) a layer of water the layer of acetic acid
Complete the following chemical equations : C2H5OH + Na →
Give the common names and IUPAC names of the following compounds of HCOOH.
How would you distinguish experimentally between an alcohol and carboxylic acid on the basis of a chemical property?
A neutral organic compound is warmed with some ethanoic acid and a little of conc. H2SO4. Vapours having sweet smell (fruity smell) are evolved. What type of functional group is present in this organic compound?
Name the functional group present in the following compound:
HCOOH
Acetic acid smells like:
(1) a banana
(2) vinegar
(3) an orange
(4) a lemon
What is thr boilng point of acetic acid?
How Will You Carry Out the Following Conversions?
Ehane to acitic acid
Fill in the blank with appropriate word/words.
Vinegar is a solution of about ________per cent ________in water.
Write a balanced equation for the following:
Write the equation for the preparation of ethylene from ethyl alcohol.
Ethanoic acid _________.
Choose the correct alternative and rewrite the following:
Some acetic acid is treated with solid NaHCO3, the resulting solution will be _________________.
Ethanoic acid is also known as which of these?
Which of the following substance produces brisk effervescence with baking soda solution?
The reaction between acetic acid and sodium carbonate is shown in the following figure.
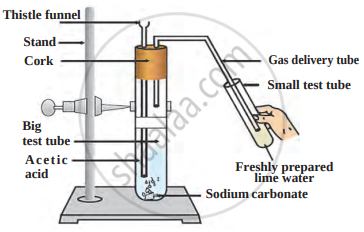
Answer the questions with the help of a diagram.
- Which gas does come out as effervescence in the big test tube?
- What is the colour change in the lime water present in the small test tube?
- Write the related reaction.
Give the balanced chemical equation of the following reaction:
Oxidation of ethanol by acidified potassium dichromate.
Write the chemical equation for the ethanol to ethanoic acid of an oxidation reaction.
Give the balanced chemical equation of the following reaction:
Neutralization of NaOH with ethanoic acid.
