Advertisements
Advertisements
प्रश्न
Write balanced chemical equations to show : The oxidizing action of conc. Sulphuric acid on carbon
Write balanced equations for Action of concentrated sulphuric acid on carbon
उत्तर १
C + 2H2SO4  CO2 + 2H2O + 2SO2 ↑
CO2 + 2H2O + 2SO2 ↑
उत्तर २
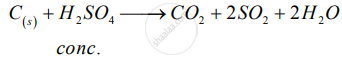
APPEARS IN
संबंधित प्रश्न
Write balanced chemical equations to show how SO3 is converted to Sulphuric acid in the contact process.
Identify the acid in the case:
The acid on mixing with lead nitrate solution produces a white precipitate, which is insoluble even on heating.
Give balanced chemical equations for the action of sulphuric acid on the following:
Potassium hydrogen carbonate.
Name the products formed when hot and concentrated sulphuric acid reacts with Sulphur.
Name the products formed when hot and concentrated sulphuric acid reacts with Carbon.
How are the following conversion brought about? Give equation and condition:
Sucrose to sugar charcoal.
How are the following conversion brought about? Give equation and condition:
Oxalic acid to carbon monoxide.
Write balanced equation for the reaction between iron and dilute sulphuric acid.
In this question, you required to supply the word (or words) that will make the sentence correct. Rewrite the copper statement.
Copper sulphate crystals are dehydrate by sulphuric acid.
An acid obtained from concentrated nitric acid on reaction with Sulphur ______.
