Advertisements
Advertisements
Questions
Write balanced chemical equations to show : The oxidizing action of conc. Sulphuric acid on carbon
Write balanced equations for Action of concentrated sulphuric acid on carbon
Solution 1
C + 2H2SO4  CO2 + 2H2O + 2SO2 ↑
CO2 + 2H2O + 2SO2 ↑
Solution 2
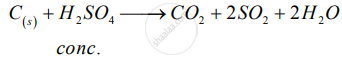
APPEARS IN
RELATED QUESTIONS
Give one equation to show the following properties of sulphuric acid:
Acidic nature
Why is Concentrated sulphuric acid kept in air tight bottles?
Give reason for the following:
When concentrated sulphuric acid is added to blue crystalline copper sulphate, it turns powdery white.
Describe the reaction that show
Dilute sulphuric acid behaves as dibasic acid.
The following statement is correct only under certain conditions. Rewrite the statement including the appropriate conditions.
Oxalic acid reacts with sulphuric acid to produce carbon monoxide and carbon dioxide.
Sulphuric acid can be used to prepare a number of gases in the laboratory. Write balanced equation for the reaction from which the folllowing gas are obtained, using dilute sulphuric acid as one of the reactant : Carbon dioxide
What do you observe when barium chloride solution is added to dilute sulphuric acid ?
Give a chemical test to distinguish between dilute sulphuric acid and dilute hydrochloric acid, (using lead nitrate solution).
Rewrite the following statement by adding the correct word, as shown in the example:
|
Example: Given Statement: Ammonia changes moist red litmus to blue. Correct Statement: Aqueous ammonia changes moist red litmus to blue. |
Sulphuric acid acts as a dehydrating agent.
Hydrogen chloride gas is prepared in the laboratory by the action of concentrated sulphuric acid on sodium chloride.
State the method of collection of the gas formed above.
