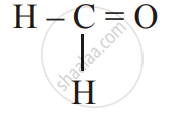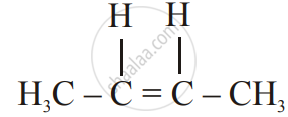(English Medium)
Academic Year: 2017-2018
Date: March 2018
Advertisements
The salt solution which does not react with ammonium hydroxide is :
Calcium Nitrate
Zinc Nitrate
Lead Nitrate
Copper Nitrate
Chapter: [0.03] Study of Acids, Bases and Salts
The organic compound which undergoes substitution reaction is :
(A) `C_2H_2`
(B) `C_2H_4`
(C) `C_10H_18`
(D) `C_2H_6`
Chapter: [0.09] Organic Chemistry
The electrolysis of acidified water is an example of :
(A) Reduction
(B) Oxidation
(C) Redox reaction
(D) Synthesis
Chapter: [0.06] Electrolysis
The IUPAC name of dimethyl ether is
(A) Ethoxy methane
(B) Methoxy methane
(C) Methoxy ethane
(D) Ethoxy ethane
Chapter:
The catalyst used in the contact process is
(A) Copper
(B) Iron
(C) Vanadium pentoxide
(D) Manganese dioxide
Chapter: [0.09] Organic Chemistry
The energy released when an electron is added to a neutral gaseous isolated atom to form a negatively charged iron.
Chapter: [0.01] Periodic Table, Periodic Properties and Variations of Properties
A process of formation of ions from molecules which are not in the ionic state
Chapter: [0.01] Periodic Table, Periodic Properties and Variations of Properties
Give one word/a phase/term/process for the following statement:
The tendency of an element to form chains of identical atoms.
Chapter: [0.09] Organic Chemistry
The property by which certain hydrated salts, when left exposed to the atmosphere, lose their water of crystallization and crumble into powder.
Chapter: [0.03] Study of Acids, Bases and Salts
The process by which sulphide ore is concentrated.
Chapter: [0.07] Metallurgy
Write balanced chemical equations to show : The oxidizing action of conc. Sulphuric acid on carbon
Chapter: [0.084] Sulphuric Acid
Write a balanced chemical equation for the reaction of sodium hydroxide solution with iron (III) chloride solution
Chapter: [0.03] Study of Acids, Bases and Salts
Write a balanced chemical equation for the action of heat on aluminium hydroxide.
Chapter: [0.07] Metallurgy
Write a balanced chemical equation for the reaction of zinc with potassium hydroxide solution.
Chapter: [0.07] Metallurgy
Write a balanced chemical equation for Action of dilute hydrochloric acid on magnesium sulphite.
Chapter: [0.03] Study of Acids, Bases and Salts
Give the IUPAC name of the following:
\[\begin{array}{cc}
\phantom{}\ce{H}\phantom{...}\ce{H}\phantom{...}\ce{H}\phantom{..}\\
\phantom{}|\phantom{....}|\phantom{....}|\phantom{..}\\
\ce{H - C - C - C - OH}\\
\phantom{}|\phantom{....}|\phantom{....}|\phantom{..}\\
\ce{\phantom{}H\phantom{...}H\phantom{...}H\phantom{..}}
\end{array}\]
Chapter:
Write the structural formula of the two isomers of butane
Chapter:
State one relevant observation for the following.
Lead nitrate solution is treated with sodium hydroxide solution dropwise till it is excess.
Chapter: [0.04] Analytical Chemistry
Advertisements
State one relevant observation for the following:
At the anode, when molten lead bromide is electrolyzed using graphite electrodes.
Chapter: [0.06] Electrolysis
State your observation when dilute hydrochloric acid is added to a lead nitrate solution and the mixture is heated.
Chapter: [0.081] Hydrogen Chloride
State one relevant observation for the following:
Anhydrous calcium chloride is exposed to air for some time.
Chapter: [0.03] Study of Acids, Bases and Salts
State your observations when Barium chloride solution is mixed with Sodium Sulphate Solution.
Chapter: [0.04] Analytical Chemistry
Why do ionic compounds have high melting points?
Chapter:
Give a reason for Inert gases do not form ions.
Chapter: [0.02] Chemical Bonding
Give a reason for Ionisation potential increases across a period, from left to right
Chapter: [0.01] Periodic Table, Periodic Properties and Variations of Properties
Give a reason for Alkali metals are good reducing agents.
Chapter: [0.07] Metallurgy
Give a reason for Conductivity of dilute hydrochloric acid is greater than that of acetic acid
Chapter: [0.09] Organic Chemistry
Name the gas that is produced in the given cases :
Sulphur is oxidized by concentrated nitric acid.
Chapter: [0.083] Nitric Acid
Name the gas that is produced in the given cases :
An action of dilute hydrochloride acid on sodium sulphide.
Chapter: [0.081] Hydrogen Chloride
Name the gas that is produced in the given case:
The action of cold and dilute nitric acid on copper
Chapter: [0.083] Nitric Acid
Name the gas that is produced in each of the given case :
At the anode during the electrolysis of acidified water.
Chapter: [0.06] Electrolysis
Name the gas that is produced in the given case:
The reaction of ethanol and sodium.
Chapter: [0.07] Metallurgy
Ionic or electrovalent compounds do not conduct electricity in their _______ state. (fused/solid)
Chapter:
Electrolysis of aqueous sodium chloride solution will form ________ at the cathode. (Hydrogen gas / Sodium metal)
Chapter: [0.06] Electrolysis
Dry hydrogen chloride gas can be collected by ________ displacement of air. (downward / upward)
Chapter: [0.081] Hydrogen Chloride
The most common ore of iron is ________. (Calcium / Haematite)
Chapter: [0.07] Metallurgy
The salt prepared by the method of direct combination is _______.
Iron (II) chloride \[\ce{(FeCl2)}\]
Iron (III) chloride \[\ce{(FeCl3)}\]
Chapter: [0.03] Study of Acids, Bases and Salts
What do you understand by a lone pair of electrons?
Chapter:
Draw the electron dot diagram of Hydionium ion (H = 1; O = 8)
Chapter:
Advertisements
In Period 3 of the Periodic Table, element B is placed to the left of element A. On the basis of this information, choose the correct word from the option to complete the following statement:
The element B would have ______ metallic character than A.
lower
higher
Chapter: [0.01] Periodic Table, Periodic Properties and Variations of Properties
In Period 3 of the Periodic Table, element B is placed to the left of element A. On the basis of this information, choose the correct word from the option to complete the following statement:
The element A would probably have ______ electron affinity than B.
lesser
higher
Chapter: [0.01] Periodic Table, Periodic Properties and Variations of Properties
In Period 3 of the Periodic Table, element B is placed to the left of element A. On the basis of this information, choose the correct word from the option to complete the following statement:
The element A would have ______ atomic size than B.
greater
smaller
Chapter: [0.01] Periodic Table, Periodic Properties and Variations of Properties
Copy and complete the following table which refers to the conversion of ions to neutral particles.
| Conversion | Ionic equation | Oxidation / Reduction |
| Chloride ion to chlorine molecule | 1)_________ | 2)_________ |
| Lead (II) ion to lead | 3)_________ | 4)_________ |
Chapter: [0.06] Electrolysis
Write the balanced chemical equation to prepare ammonia gas in the laboratory by using an alkali.
Chapter: [0.07] Metallurgy
State why concentrated sulphuric acid is not used for drying ammonia gas
Chapter: [0.07] Metallurgy
Why is ammonia gas not collected over water?
Chapter: [0.07] Metallurgy
Name the acid used for the preparation of hydrogen chloride gas in the laboratory. Why is this particular acid preferred to other acids?
Chapter: [0.081] Hydrogen Chloride [0.1] Practical Work
Write the balanced chemical equation for the laboratory preparation of hydrogen chloride gas.
Chapter: [0.081] Hydrogen Chloride [0.1] Practical Work
For the preparation of hydrochloric acid in the laboratory:
Why is the direct absorption of hydrogen chloride gas in water not feasible?
Chapter: [0.081] Hydrogen Chloride [0.1] Practical Work
For the preparation of hydrochloric acid in the laboratory:
What arrangement is done to dissolve hydrogen chloride gas in water?
Chapter: [0.081] Hydrogen Chloride
For the electro-refining of copper: What is the cathode made up of?
Chapter: [0.07] Metallurgy
For the electro-refining of copper: Write the reaction that takes place at the anode
Chapter: [0.07] Metallurgy
The percentage composition of a gas is Nitrogen 82.35%, Hydrogen 17.64%. Find the empirical formula of the gas. [N = 14, H = 1]
Chapter: [0.05] Mole Concept and Stoichiometry
Aluminium carbide reacts with water according to the following equation :
`Al_4C_3 + 12H_2O-> 4Al(OH)_3 + 3CH_4`
1)What mass of aluminium hydroxide is formed from 12g of aluminium carbide?
2) What volume of methane at s.t.p. is obtained from 12g of aluminium carbide?
[Relatively molecular weight of `Al_4Cl_3 = 144; Al(OH)_3 = 78]`
Chapter: [0.05] Mole Concept and Stoichiometry
If 150 cc of gas A contains X molecules, how many molecules of gas B will be present in 75 cc of B?. The gases A and B are under the same conditions of temperature and pressure.
Chapter: [0.05] Mole Concept and Stoichiometry
Name the law om which same conditions of temperature and pressure
Chapter: [0.05] Mole Concept and Stoichiometry
State the main components of the following alloys:
Brass
Chapter: [0.07] Metallurgy
State the main components of the following alloys:
Duralumin
Chapter: [0.07] Metallurgy
Complete the following table which relates to the homologous series of hydrocarbons.
|
General Formula |
IUPAC name of the homologous series | Characteristic bond type | IUPAC name of the first member of the series |
| `C_nH_(2n-2)` | (A)________ | (B)______ | (C)______ |
| C_nH_(2n+1) | (B)________ | (E)______ | (F)______ |
Chapter: [0.09] Organic Chemistry
Name the most common ore of the metal aluminium from which the metal is extracted. Write the chemical formula of the ore.
Chapter: [0.07] Metallurgy
Name the process by which impure ore of aluminium gets purified by using the concentrated solution of an alkali.
Chapter: [0.07] Metallurgy
Write the equation for the formation of aluminium at the cathode during the electrolysis of alumina.
Chapter: [0.07] Metallurgy
A compound X (having the vinegar-like smell) when treated with ethanol in the presence of the acid Z, gives a compound Y which has a fruity smell.
The reaction is:
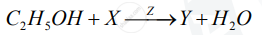
1) Identify Y and Z.
2) Write the structural formula of X.
3) Name the above reaction.
Chapter: [0.04] Analytical Chemistry
Ethane burns in oxygen to form CO2 and H2O according to the equation:
`2C_2H_6+7O_2 -> 4CO_2 + 6H_2O`
If 1250 cc of oxygen is burnt with 300 cc of ethane.
Calculate:
1) the volume of `CO_2` formed
2) the volume of unused `O_2`
Chapter: [0.05] Mole Concept and Stoichiometry
Three solutions P, Q and R have pH value of 3.5, 5.2 and 12.2 respectively. Which one of these is a:
1) Weak acid?
2) Strong alkali?
Chapter: [0.03] Study of Acids, Bases and Salts
Give a chemical test to distinguish between the given pairs of chemicals:
Lead nitrate solution and Zinc nitrate solution
Chapter: [0.04] Analytical Chemistry
Give a chemical test to distinguish between the given pairs of chemicals:
Sodium chloride solution and Sodium nitrate solution
Chapter: [0.081] Hydrogen Chloride
Write a balanced equation for the preparation of the following salts:
Copper sulphate from Copper Carbonate.
Chapter: [0.03] Study of Acids, Bases and Salts
Write a balanced equation for the preparation of the following salts:
Zinc carbonate from Zinc sulphate
Chapter: [0.03] Study of Acids, Bases and Salts
What is the type of salt formed when the reactants are heated at a suitable temperature for the preparation of Nitric acid?
Chapter: [0.083] Nitric Acid
Which property of sulphuric acid is shown by the reaction of the concentrated sulphuric acid with Ethanol?
Chapter: [0.084] Sulphuric Acid
Which property of sulphuric acid is shown by the reaction of the concentrated sulphuric acid with Carbon?
Chapter: [0.084] Sulphuric Acid
Other Solutions
Submit Question Paper
Help us maintain new question papers on Shaalaa.com, so we can continue to help studentsonly jpg, png and pdf files
CISCE previous year question papers ICSE Class 10 Chemistry with solutions 2017 - 2018
Previous year Question paper for CISCE ICSE Class 10 -2018 is solved by experts. Solved question papers gives you the chance to check yourself after your mock test.
By referring the question paper Solutions for Chemistry, you can scale your preparation level and work on your weak areas. It will also help the candidates in developing the time-management skills. Practice makes perfect, and there is no better way to practice than to attempt previous year question paper solutions of CISCE ICSE Class 10 .
How CISCE ICSE Class 10 Question Paper solutions Help Students ?
• Question paper solutions for Chemistry will helps students to prepare for exam.
• Question paper with answer will boost students confidence in exam time and also give you an idea About the important questions and topics to be prepared for the board exam.
• For finding solution of question papers no need to refer so multiple sources like textbook or guides.