Advertisements
Advertisements
Question
For the preparation of hydrochloric acid in the laboratory:
Why is the direct absorption of hydrogen chloride gas in water not feasible?
Solution
The reaction is highly exothermic.
APPEARS IN
RELATED QUESTIONS
Certain blank spaces are left in the following table and these are labelled as A, B, C, D
and E. Identify each of them
| Lab preparation of | Reactants used | Products formed | Drying Agent | Method of collection |
|
| 1 | HCl gas | NaCl + H2SO4 |  |
conc. H2SO4 | 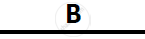 |
| 2 | NH3 gas |
|
Mg(OH)2 NH3 |
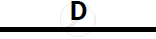 |
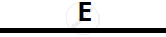 |
Identify the gas evolved and give the chemical test in the following cases
Dilute hydrochloric acid reacts with iron (II) sulphide.
The following are pertaining to the laboratory preparation of hydrogen chloride gas.
Name the drying agent used and justify your choice.
State your observation in given case When dilute hydrochloric acid is added to sodium carbonate crystals
Give the balanced equation for the laboratory preparation of hydrogen chloride gas reaction.
Explain why when the stopper of a bottle full of hydrogen chloride gas is opened, there are fumes in the air.
Explain why a solution of hydrogen chloride in water turns blue litmus red and conducts electricity, while a solution of the same gas in toluene:
- has no effect on litmus, and
- does not conduct electricity.
Explain why dry hydrogen chloride gas does not affect a dry strip of blue litmus paper but it turns red in the presence of a drop of water.
Name the gas produced when chlorine water is exposed to sunlight.
Draw a labelled diagram and explain the laboratory preparation of hydrogen chioride gas.
What are the important precautions?
Describe an experiment to prove the following:
HCI gas contains the element chlorine.
Choose the correct answer from the options given below:
Dilute hydrochloric acid solution cannot be concentrated by boiling beyond
Write the equation for the reaction which takes place in question(a).
Give one test to distinguish between the following pair of chemicals.
Iron (III) Chloride solution and copper chloride solution.
Answer the following question related to the laboratory preparation of the hydrogen chloride gas:
State the temperature required in the preparation.
