Advertisements
Advertisements
प्रश्न
Write balanced chemical equations to show : The oxidizing action of conc. Sulphuric acid on carbon
Write balanced equations for Action of concentrated sulphuric acid on carbon
उत्तर १
C + 2H2SO4  CO2 + 2H2O + 2SO2 ↑
CO2 + 2H2O + 2SO2 ↑
उत्तर २
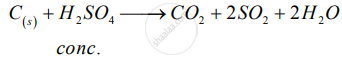
APPEARS IN
संबंधित प्रश्न
Give one equation to show the following properties of sulphuric acid:
As a non-volatile acid
Which property of sulphuric acid is shown by the reaction of the concentrated sulphuric acid with Carbon?
Name the products formed when hot and concentrated sulphuric acid reacts with Sugar.
Name the following :
The precipitate obtained by treating aqueous lead nitrate with dilute sulphuric acid.
Give reason for the following:
When concentrated sulphuric acid is added to sugar/glucose, a black mass is left behind.
Give reason for the following:
When concentrated sulphuric acid is added to blue crystalline copper sulphate, it turns powdery white.
Describe the reaction that show
Concentrated sulphuric acid is a dehydrating agent.
Why is sulphuric acid known as king of chemicals and oil vitriol ?
Name the gas released when sodium carbonate is added to a solution of sulphur dioxide.
Hydrogen chloride gas is prepared in the laboratory by the action of concentrated sulphuric acid on sodium chloride.
Give a balanced chemical equation for the above reaction.
