Advertisements
Advertisements
प्रश्न
Write Lewis symbols for the following atoms and ions: S and S2–.
उत्तर १
S and S2–
The number of valence electrons in sulphur is 6.
The Lewis dot symbol of sulphur (S) is 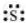
The dinegative charge infers that there will be two electrons more in addition to the six valence electrons. Hence, the Lewis dot symbol of S2– is 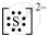
उत्तर २
`""_16"S"` = 2,8,6 ∴ Lewis symbol = 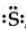 , `"S"^(2-) "ions" = `
, `"S"^(2-) "ions" = ` 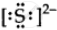
APPEARS IN
संबंधित प्रश्न
Write Lewis symbols for the following atoms and ions: Al and Al3+.
Write Lewis symbols for the following atoms and ions H and H–.
Use Lewis symbols to show electron transfer between the following atoms to form cations and anions:-
K and S
Use Lewis symbols to show electron transfer between the following atoms to form cations and anions:
Al and N
Draw Lewis dot diagram for the following.
Hydrogen (H2)
Draw Lewis dot diagram for the following.
Methane (CH4)
Draw Lewis electron dot structure of C2H6
Draw Lewis electron dot structure of C2H4
Draw Lewis electron dot structure of CF3Cl
Draw Lewis electron dot structure of SO2
Explain in brief with one example of coordinate bond
Which of the following molecules has a central atom with complete octet?
Which of the following is INCORRECT with respect to enthalpy?
In which of the following, the central atom does NOT have lone pair(s) of electrons?
Which of the following molecule does not obey octet rule?
Match the items given in Column I with examples given in Column II.
| Column I | Column II |
| (i) Hydrogen bond | (a) \[\ce{C}\] |
| (ii) Resonance | (b) \[\ce{LiF}\] |
| (iii) Ionic solid | (c) \[\ce{H2}\] |
| (iv) Covalent solid | (d) \[\ce{HF}\] |
| (e) \[\ce{O3}\] |
Assertion (A): Sodium chloride formed by the action of chlorine gas on sodium metal is a stable compound.
Reason (R): This is because sodium and chloride ions acquire octet in sodium chloride formation.
