Advertisements
Advertisements
प्रश्न
Write short notes on Acetylation
उत्तर
Acetylation - Acetylation (or ethanoylation) is the process of introducing an acetyl group into a molecule.
Aliphatic and aromatic primary and secondary amines undergo acetylation reaction by nucleophilic substitution when treated with acid chlorides, anhydrides or esters. This reaction involves the replacement of the hydrogen atom of −NH2 or > NH group by the acetyl group, which in turn leads to the production of amides. To shift the equilibrium to the right hand side, the HCl formed during the reaction is removed as soon as it is formed. This reaction is carried out in the presence of a base (such as pyridine) which is stronger than the amine.
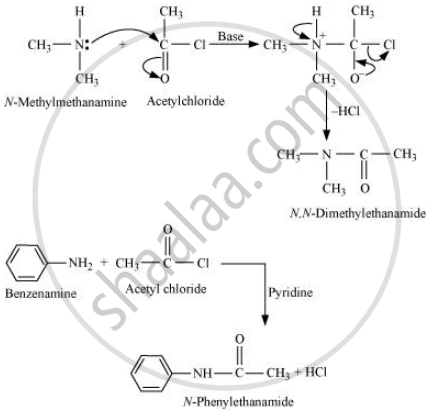
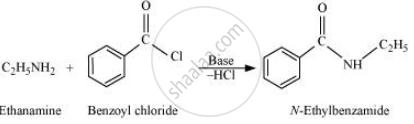
When amines react with benzoyl chloride, the reaction is also known as benzoylation.
For example,
APPEARS IN
संबंधित प्रश्न
How is chlorobenzene prepared from aniline?
What is the action of acetic anhydride on ethylamine?
What is the action of the following reagents on aniline?
Bromine water
Give reasons for the following:
Aniline does not undergo Friedel- Crafts reaction.
Write the structures of main products when aniline reacts with the following reagents :
Br2 water
Write the structures of main products when aniline reacts with the following reagents :
(CH3CO)2O/pyridine
What is the action of the following reagents on aniline?
Acetic anhydride
Illustrate the following reactions giving suitable example in each case
Acetylation of amines
How will you convert the following?
Aniline into N−phenylethanamide
In the nitration of benzene using a mixture of conc. \[\ce{H2SO4}\] and conc. \[\ce{HNO3}\], the species which initiates the reaction is ______.
What is the role of \[\ce{HNO3}\] in the nitrating mixture used for nitration of benzene?
Assertion: N, N-Diethylbenzene sulphonamide is insoluble in alkali.
Reason: Sulphonyl group attached to nitrogen atom is strong electron-withdrawing group.
When bromination of aniline is carried out by protecting – NH2. The major product is
Give reasons for the following observation:
Aniline is acetylated before nitration reaction.
Give reasons for the following observation:
Aniline does not react with methyl chloride in the presence of anhydrous AlCl3 catalyst.
How can the activating effect of the −NH2 group in aniline be controlled?
Assertion (A): Bromination of benzoic acid, gives m-bromobenzoic acid.
Reason (R): Carboxyl group increases the electron density at the meta position.
Aniline does not give Friedel-Crafts reaction. Give a reason.
