Advertisements
Advertisements
प्रश्न
Write structures of the compounds whose IUPAC names are as follows:
3-Chloromethylpentan-1-ol.
उत्तर
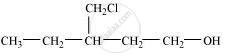
APPEARS IN
संबंधित प्रश्न
Name the following compound according to IUPAC system.
\[\begin{array}{cc}
\phantom{............}\ce{CH2OH}\\
\phantom{......}|\\
\ce{CH3 - CH2 - CH - CH - CH - CH3}\\
\phantom{......}|\phantom{............}|\phantom{.}\\
\phantom{........}\ce{CH2Cl}\phantom{......}\ce{CH3}\phantom{}
\end{array}\]
Write IUPAC name of the following compound:
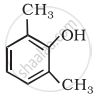
Write IUPAC name of the following compound:
C6H5 – O – C2H5
Natalite is a mixture of
(a) diethyl ether and methanol
(b) diethyl ether and ethanol
(c) dimethyl ether and methanol
(d) dimethyl ether and ethanol
What is the action of hot HI on it?
Resorcinol on distillation with zinc dust gives _________.
Isopropyl alcohol on oxidation forms:
3-methylphenol is called ____________.
The major product formed by the reaction:
\[\begin{array}{cc}
\ce{CH3CH-CH2Br ->[CH3O^-][CH3OH] is}\\
|\phantom{................}\\
\ce{CH3}\phantom{.............}
\end{array}\]
Among the following sets of reactants which one produces anisole?
Give IUPAC name of the compound given below.
\[\begin{array}{cc}
\phantom{}\ce{CH3 - CH - CH2 - CH2 - CH - CH3}\phantom{.}\\
\phantom{.........}|\phantom{...................}|\phantom{...........}\\
\phantom{..}\ce{Cl}\phantom{.................}\ce{OH}\phantom{..}
\end{array}\]
Which of the following compounds will react with sodium hydroxide solution in water?
Assertion: Addition reaction of water to but-1-ene in acidic medium yields butan-1-ol.
Reason: Addition of water in acidic medium proceeds through the formation of primary carbocation.
Explain why Lewis acid is not required in bromination of phenol?
Write chemical reactions for the following conversion:
Acetic acid into ethyl alcohol
Draw structure of the following compound.
2-Methoxypropane
Give the structures of Thiosulphuric acid and Peroxy monosulphuric acid.
Write IUPAC names of the following compounds:
\[\begin{array}{cc}
\phantom{...............}\ce{CH3}\\
\phantom{.............}|\\
\ce{CH3 - CH - CH - C - CH3}\\
|\phantom{......}|\phantom{......}|\\
\phantom{...}\ce{CH3\phantom{...}OH\phantom{...}CH3}\\
\end{array}\]
