Advertisements
Advertisements
प्रश्न
A metal carbonate X on reacting with an acid gives a gas which when passed through a solution Y gives the carbonate back. On the other hand, a gas G that is obtained at anode during electrolysis of brine is passed on dry Y, It gives a compound Z, used for disinfecting drinking water. Identity X, Y, G and Z.
उत्तर
The gas G obtained at anode during the electrolysis of brine is chlorine. The compound Z used for disinfecting drinking water is bleaching powder. It is formed on reacting chlorine with dry slaked lime i.e., Ca(OH)2. It is denoted as Y This means that the metal carbonate X is calcium carbonate. Upon heating, it evolves CO2 gas which gives back X on reacting with calcium hydroxide. The chemical reactions involved are listed :
`underset((X))("CaCO"_3) + underset("(Acid)")(2"HCl") -> "CaCl"_2 + "H"_2"O" + "CO"_2`
`underset("(Y)")("Ca"("OH")_2) + "CO"_2 -> underset ((x))("CaCO"_3) + "H"_2"O"`
`"Ca"("OH")_2 + underset("(From brine)")underset("(G)") -> underset("Bleaching Powder")underset("(Z)")("CaOCl"_2) + "H"_2"O"`
APPEARS IN
संबंधित प्रश्न
While diluting an acid, why is it recommended that the acid should be added to water and not water to the acid?
What colour do the following indicators turn when added to a base or alkali (such as sodium hydroxide)?
(a) methyl orange
Which gas is liberated when dilute hydrochloric acid reacts with sodium carbonate?
What happens when carbon dioxide gas is passed through lime water for a short time?
Write equations of the reactions involved.
You have been provided with three test-bubes. One of these test-tubes contains distilled water and the other two contain an acidic and a basic solution respectively. If you are given only blue litmus paper, how will you identify the contents of each test-tube?
What is common in all the water soluble bases (or alkalis)?
Answer the following question.
Blue litmus solution is added to two test tubes A and B containing dilute HCl and NaOH solution respectively. In which test tube a colour change will be observed? State the colour change and give its reason.
In the experimental set-up to show that "the germinating seeds give out carbon dioxide", answer the following questions:
(i) Why do we keep the conical flask airtight?
(ii) Name the substance kept in the small test tube inside the conical flask. Write its role.
(iii) Why does water rise in the delivery tube?
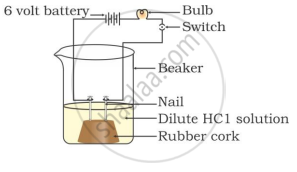
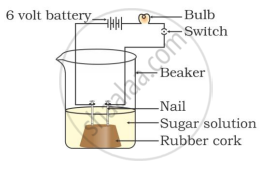
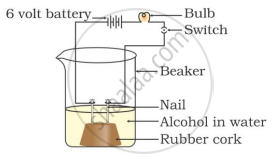
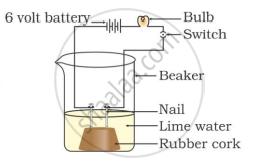
In which of the following setups would the bulb glow?
Which of the following phenomena occur when a small amount of acid is added to water?
- Ionisation
- Neutralisation
- Dilution
- Salt formation