Advertisements
Advertisements
प्रश्न
A student takes 2 mL acetic acid in a dry test tube and adds a pinch of sodium hydrogen carbonate to it. He makes the following observations:
I. A colourless and odourless gas evolves with a brisk effervescence.
II. The gas turns lime water milky when passed through it.
III. The gas burns with an explosion when a burning splinter is brought near it.
IV. The gas extinguishes the burning splinter that is brought near it.
The correct observations are:
(A) I, II, and III
(B) II, III and IV
(C) III, IV and I
(D) IV, I and II
उत्तर
(D)
When 2 mL acetic acid is taken in a dry test tube and a pinch of sodium hydrogen carbonate is added to it, a colourless and odourless gas evolves with a brisk effervescence which is CO2.
When CO2 is passed through lime water it turns lime water milky. 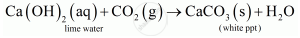
CO2 also extinguishes the burning splinter when it is brought near it.
APPEARS IN
संबंधित प्रश्न
What are esters ?
Write the formulae of methanoic acid.
Which of the following will give brisk effervescence with sodium hydrogen carbonate and why?
CH3COOH, CH3CH2OH
If you take a pinch of sodium hydrogen carbonate powder in a test-tube and add drop-by-drop acetic acid to it, what would you observe immediately? List any two main observations.
Name the functional group present in the following compound:
HCOOH
State how the following conversions can be carried out:
Ethyl chloride to Ethyl alcohol
Observe the figure and write the answers to the following questions.

- Write the name of the reaction shown in the following figure.
- Write the above chemical reaction in the form of a balanced equation.
- Write the name of the product produced in the above reaction, write a use.
- Write the name of the catalyst used in the above reaction.
Give the balanced chemical equation of the following reaction:
Neutralization of NaOH with ethanoic acid.
In the presence of the acid catalyst, ethanoic acid reacts with ethanol and ______ ester is produced.
Write the chemical equation for the ethanol to ethanoic acid of an oxidation reaction.
