Advertisements
Advertisements
प्रश्न
Among Zn and Cu, which would occur more readily in nature as metal and which as an ion?
उत्तर
Since the electron-releasing tendency of zinc is more than that of copper, therefore, copper would occur more readily in nature as a metal and zinc as an ion.
APPEARS IN
संबंधित प्रश्न
What happens if external potential applied becomes greater than E°cell of electrochemical cell?
Can you store copper sulphate solutions in a zinc pot?
Construct a labelled diagram for the following cell:
`Zn|Zn^(2+)(1M)||H^+(1M)|H_(2(g,1atm))|Pt`
How many faradays of electricity are required to produce 6 g of Mg from MgCl2?
E°cell for the given redox reaction is 2.71 V
Mg(s) + Cu2+ (0.01 M) → Mg2+ (0.001 M) + Cu(s)
Calculate Ecell for the reaction. Write the direction of flow of current when an external opposite potential applied is
(i) less than 2.71 V and
(ii) greater than 2.71 V
Reduction potential of two metals M1 and M2 are \[\ce{E^0_{{M_1^{2+}|M_1}}}\] = −2.3 V and \[\ce{E^0_{{M_2^{2+}|M_2}}}\] = 0.2 V. Predict which one is better for coating the surface of iron.
Given: \[\ce{E^0_{{Fe^{2+}|Fe}}}\] = −0.44 V
How will the pH of brine (aq. \[\ce{NaCl}\] solution) be affected when it is electrolysed?
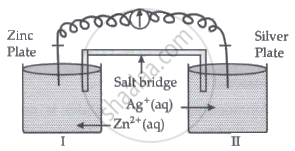
Read the passage given below and answer the questions that follow:
|
Oxidation-reduction reactions are commonly known as redox reactions. They involve transfer of electrons from one species to another. In a spontaneous reaction, energy is released which can be used to do useful work. The reaction is split into two half-reactions. Two different containers are used and a wire is used to drive the electrons from one side to the other and a Voltaic/Galvanic cell is created. It is an electrochemical cell that uses spontaneous redox reactions to generate electricity. A salt bridge also connects to the half-cells. The reading of the voltmeter gives the cell voltage or cell potential or electromotive force. If \[\ce{E^0_{cell}}\] is positive the reaction is spontaneous and if it is negative the reaction is non-spontaneous and is referred to as electrolytic cell. Electrolysis refers to the decomposition of a substance by an electric current. One mole of electric charge when passed through a cell will discharge half a mole of a divalent metal ion such as Cu2+. This was first formulated by Faraday in the form of laws of electrolysis.
|
- Is silver plate the anode or cathode? (1)
- What will happen if the salt bridge is removed? (1)
- When does electrochemical cell behaves like an electrolytic cell? (1)
- (i) What will happen to the concentration of Zn2+ and Ag+ when Ecell = 0. (1)
(ii) Why does conductivity of a solution decreases with dilution? (1)
OR
The molar conductivity of a 1.5 M solution of an electrolyte is found to be 138.9 S cm2mol-1. Calculate the conductivity of this solution. (2)
Calculate the λ0m for Cl- ion from the data given below:
∧0m MgCl2 = 258.6 Scm2 mol-1 and λ0m Mg2+ = 106 Scm2 mol-1
What is an electrochemical cell? What does it consist of?