Advertisements
Advertisements
प्रश्न
An ideal gas expands from 100 cm3 to 200 cm3 at a constant pressure of 2.0 × 105 Pa when 50 J of heat is supplied to it. Calculate (a) the change in internal energy of the gas (b) the number of moles in the gas if the initial temperature is 300 K (c) the molar heat capacity Cp at constant pressure and (d) the molar heat capacity Cv at constant volume.
उत्तर
Initial volume of the gas, V1 = 100 cm3
Final volume = V2 = 200 cm3
Pressure = 2 × 105 Pa
Heat supplied, dQ = 50 J
(a) According to the first law of thermodynamics,
dQ = dU + dW
dW = PΔV = 2 × 105 × (200 -100) × 10-6 = 20
Initial volume of the gas, V1 = 100 cm3
Final volume = V2 = 200 cm3
Pressure = 2 × 105 Pa
Heat supplied, dQ = 50 J
(a) According to the first law of thermodynamics,
dQ = dU + dW
`"U" = 3/2 "n""R""T"`
`30 = n xx 3/2 xx 8.3 xx 300`
`=> n = 2/(83 xx3) = 2/249 = 0.008`(c) Also,
dU = nCvdT
`=> "C"_"V" = ("d""U")/ ("n""d""T") = 30 /(0.008 xx 300) = 12.5`
Cp = Cv + R = 12.5 + 8.3 = 20.8 J/mol-K
(d) Cv = 12.5 J/mol-K
APPEARS IN
संबंधित प्रश्न
The specific heat capacity of water is
Can we define specific heat capacity at constant temperature?
Can we define specific heat capacity for an adiabatic process?
Show that the slope of the p−V diagram is greater for an adiabatic process compared to an isothermal process.
Can two states of an ideal gas be connected by an isothermal process as well as an adiabatic process?
Three identical adiabatic containers A, B and C contain helium, neon and oxygen, respectively, at equal pressure. The gases are pushed to half their original volumes.
(a) The final temperatures in the three containers will be the same.
(b) The final pressures in the three containers will be the same.
(c) The pressures of helium and neon will be the same but that of oxygen will be different.
(d) The temperatures of helium and neon will be the same but that of oxygen will be different.
A mixture contains 1 mole of helium (Cp = 2.5 R, Cv = 1.5 R) and 1 mole of hydrogen (Cp= 3.5 R, Cv = 2.5 R). Calculate the values of Cp, Cv and γ for the mixture.
In Joly's differential steam calorimeter, 3 g of an ideal gas is contained in a rigid closed sphere at 20°C. The sphere is heated by steam at 100°C and it is found that an extra 0.095 g of steam has condensed into water as the temperature of the gas becomes constant. Calculate the specific heat capacity of the gas in J g−1 K−1. The latent heat of vaporisation of water = 540 cal g−1
Air (γ = 1.4) is pumped at 2 atm pressure in a motor tyre at 20°C. If the tyre suddenly bursts, what would be the temperature of the air coming out of the tyre? Neglect any mixing with the atmospheric air.
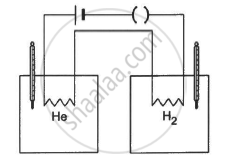
The figure shows two vessels with adiabatic walls, one containing 0.1 g of helium (γ = 1.67, M = 4 g mol−1) and the other containing some amount of hydrogen (γ = 1.4, M = 2 g mol−1). Initially, the temperatures of the two gases are equal. The gases are electrically heated for some time during which equal amounts of heat are given to the two gases. It is found that the temperatures rise through the same amount in the two vessels. Calculate the mass of hydrogen.
The speed of sound in hydrogen at 0°C is 1280 m s−1. The density of hydrogen at STP is 0.089 kg m−3. Calculate the molar heat capacities Cp and Cv of hydrogen.
Standing waves of frequency 5.0 kHz are produced in a tube filled with oxygen at 300 K. The separation between the consecutive nodes is 3.3 cm. Calculate the specific heat capacities Cp and Cv of the gas.
Molar specific heat of water is C = 74.7 J/mol K, its value in cal/g K is ______.
An engine takes in 5 moles of air at 20°C and 1 atm, and compresses it adiabatically to `1/10^"th"` of the original volume. Assuming air to be a diatomic ideal gas made up of rigid molecules, the change in its internal energy during this process comes out to be X kJ. The value of X to the nearest integer is ______.
A diatomic molecule can be modelled as two rigid balls connected with spring such that the balls can vibrate with respect to centre of mass of the system (spring + balls). Consider a diatomic gas made of such diatomic molecule. If the gas performs 20 Joule of work under isobaric condition, then heat given to the gas is ______ J.
If at same temperature and pressure, the densities for two diatomic gases are respectively d1 and d2 then the ratio of velocities of sound in these gases will be ______.
