Advertisements
Advertisements
प्रश्न
An organic compound 'A' with the molecular formula C4H8O2 undergoes acid hydrolysis to form two compounds 'B' and 'C'. Oxidation of 'C' with acidified potassium permanganate also produces 'B'. Sodium salt of 'B' on heating with soda lime gives methane.
- Identify 'A', 'B' and 'C'.
- Out of 'B' and 'C', which will have higher boiling point? Give reason.
उत्तर
- A = C4H8O2 [CH3COOC2H5] ester Reactions involved are
\[\ce{\underset{A}{CH3-COOC2H5} + H2O ->[dil H2SO4] \underset{B}{CH3COOH} + \underset{C}{CH3CH2OH}}\]
Then
\[\ce{\underset{C}{CH3CH2OH} ->[KMnO4][{[O]}] \underset{B}{CH3COOH}}\]
So A = CH3COOC2H5, B = CH3COOH, C = CH3CH2OH - B has a higher boiling point than C. Because of their propensity to generate intermolecular H-bonds, carboxylic acids have higher boiling temperatures than alcohols.
APPEARS IN
संबंधित प्रश्न
A and B are two functional isomers of compound C3H6O.On heating with NaOH and I2, isomer B forms yellow precipitate of iodoform whereas isomer A does not form any precipitate. Write the formulae of A and B.
Propanal and Propanone
Which sugar does not reduce Fehling's solution?
Solvent used for dewaxing of petroleum products are
Acetone and acetaldehyde are differentiated by
Acetaldehyde cannot show?
Ammonical silver nitrate solution is called
Write chemical test to distinguish between the following compounds:
Phenol and Benzoic acid
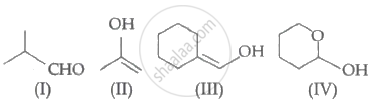
Which among the above compound/s does/do not form Silver mirror when treated with Tollen's reagent?
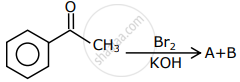
The major products formed in the following reaction sequence A and B are: