Advertisements
Advertisements
प्रश्न
An organic compound 'A' with the molecular formula C4H8O2 undergoes acid hydrolysis to form two compounds 'B' and 'C'. Oxidation of 'C' with acidified potassium permanganate also produces 'B'. Sodium salt of 'B' on heating with soda lime gives methane.
- Identify 'A', 'B' and 'C'.
- Out of 'B' and 'C', which will have higher boiling point? Give reason.
उत्तर
- A = C4H8O2 [CH3COOC2H5] ester Reactions involved are
\[\ce{\underset{A}{CH3-COOC2H5} + H2O ->[dil H2SO4] \underset{B}{CH3COOH} + \underset{C}{CH3CH2OH}}\]
Then
\[\ce{\underset{C}{CH3CH2OH} ->[KMnO4][{[O]}] \underset{B}{CH3COOH}}\]
So A = CH3COOC2H5, B = CH3COOH, C = CH3CH2OH - B has a higher boiling point than C. Because of their propensity to generate intermolecular H-bonds, carboxylic acids have higher boiling temperatures than alcohols.
APPEARS IN
संबंधित प्रश्न
Predict the products of the following reactions :
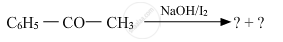
Give a simple chemical test to distinguish between the following pair of compounds:
Phenol and Benzoic acid
Give a simple chemical test to distinguish between the following pair of compounds:
Pentan-2-one and Pentan-3-one
Complete the synthesis by giving missing starting material, reagent or product.
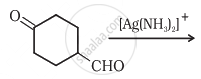
Alkenes decolourise bromine water in presence of CCl4 due to formation of ______.
Which of the following compounds will give butanone on oxidation with alkaline \[\ce{KMnO4}\] solution?
Oxidation of ketones involves carbon-carbon bond cleavage. Name the products formed on oxidation of 2, 5-dimethylhexan-3-one.
Write chemical test to distinguish between the following compounds:
Phenol and Benzoic acid
An organic compound neither reacts with neutral ferric chloride solution nor with Fehling solution. It however, reacts with Grignard reagent and gives positive iodoform test. The compound is:
Choose the reaction which is not possible:
