Advertisements
Advertisements
प्रश्न
Answer the following.
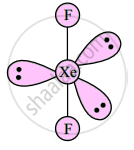
Draw structures of XeF2
उत्तर
Linear
APPEARS IN
संबंधित प्रश्न
Answer the following.
Give one example showing reducing property of ozone.
What happens when lead sulfide reacts with ozone \[\ce{O3}\]?
Answer the following.
What happens when nitric oxide reacts with ozone.
Answer the following.
Draw structures of XeF6
Answer the following.
How are xenon fluorides XeF2, XeF4 and XeF6 obtained? Give suitable reactions.
Answer the following.
Describe the structure of the Ozone. Give two uses of ozone.
O2 molecule is ______.
The number of covalent bonds present in sulfuric acid:
What is the O−S−O bond angle in SO2?
Complete the following reaction:
\[\ce{SO_{2(g)} + Cl_{2(g)} ->[Charcoal]}\] ?
Select the INCORRECT statement.
Identify the INCORRECT match.
Which of the following molecule does not contain oxygen?
Which of the following is also called as nitrogen sesquioxide?
Find the CORRECT statement.
\[\ce{I2_{(g)} + H2S_{(g)} -> 2HI_{(g)} + S_{(s)}}\]
In resonance hybrid of ozone molecule, O-O bond length is ____________.
Which among the following oxides is amphoteric in nature?
Mark the oxide which is amphoteric in character.
What will be the formula of an oxide of iodine (atomic mass = 127) which contains 25.4 g of iodine and 8g of oxygen?
Give reason:
Bleaching action of ozone is also called dry bleach.
Write balanced equation of a reaction in which ozone reduces hydrogen peroxide.
Write the reaction of the following with concentrated H2SO4:
NaCl
Calculate the oxidation state of S in oleum
Electrolytic method of preparation of dioxygen.
Industrial method of preparation of dioxygen.
