Advertisements
Advertisements
प्रश्न
Answer the following question.
What is the basic structural difference between glucose and fructose?
उत्तर
Both Glucose and Fructose are hexose sugars with six carbon atoms but Glucose is an aldohexose and fructose is ketohexose which means the functional group present in glucose is an aldehyde and the functional group in fructose is a ketone.
\[\begin{array}{cc}
\phantom{..}\ce{O}\phantom{..}\ce{H}\phantom{.}\ce{OH}\phantom{.}\ce{OH}\phantom{}\\
\phantom{}||\phantom{...}|\phantom{...}|\phantom{...}|\phantom{.}\\
\ce{CH2OH-C-C-C-C-CH2OH}\\
\phantom{....}|\phantom{...}|\phantom{...}|\phantom{}\\
\phantom{....}\ce{HO}\phantom{..}\ce{H}\phantom{..}\ce{H}\phantom{..}\\
\text{D-fructose}
\end{array}\]
\[\begin{array}{cc}
\phantom{}\ce{OH}\phantom{..}\ce{H}\phantom{.}\ce{OH}\phantom{.}\ce{OH}\phantom{....}\\
\phantom{.}|\phantom{...}|\phantom{...}|\phantom{...}|\phantom{....}\\
\ce{CHO-C-C-C-C-CH2OH}\\
\phantom{}|\phantom{...}|\phantom{...}|\phantom{...}|\phantom{...}\\
\phantom{}\ce{H}\phantom{.}\ce{HO}\phantom{.}\ce{H}\phantom{..}\ce{H}\phantom{..}\\
\text{D-glucose}
\end{array}\]
संबंधित प्रश्न
Maltose is a
(a) Polysaccharide
(b) Disaccharide
(c) Trisaccharide
(d) Monosaccharide
What do you observe when glucose solution is heated with Tollen’s reagent?
Write the reactions involved when D-glucose is treated with the following reagent:
H2N-OH
Choose the appropriate answer(s) for the below representation from the options given
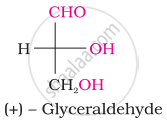
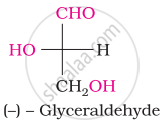
Glucose reacts with acetic anhydride to form ______.
Which of the following properties of glucose cannot be explained by its open chain structure?
(i) Glucose does not form hydrogen sulphite with NaHSO3.
(ii) On oxidation with HNO3 glucose gives saccharic acid.
(iii) Glucose is found to exist in two different crystalline forms which are named as α and β.
Three structures are given below in which two glucose units are linked. Which of these linkages between glucose units are between C1 and C4 and which linkages are between C1 and C6?
| (I) |  |
| (II) | 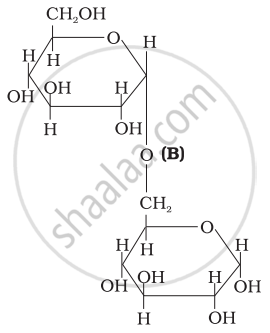 |
| (III) | 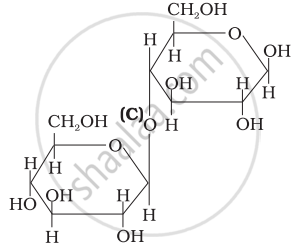 |
Why does compound (A) given below not form an oxime?
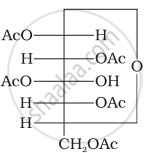
(A)
Assertion: D (+) – Glucose is dextrorotatory in nature.
Reason: ‘D’ represents its dextrorotatory nature.
Account for the following:
What happens when D – glucose is treated with the following reagents
HNO3
