Advertisements
Advertisements
प्रश्न
Calculate the degree of dissociation (α) of acetic acid if its molar conductivity (Λm) is 39.05 S cm2 mol−1.
Given λ°(H+) = 349.6 S cm2 mol−1 and λ°(CH3COO−) = 40.9 S cm2 mol−1
उत्तर
The degree of dissociation of a weak electrolyte is given by the following relation
α = Λm / Λ0 ... Equation (1)
where α is the degree of dissociation of the electrolyte, Λm is the molar conductivity and Λ0 is the molar conductivity at infinite dilution.
We are given that the ionic molar conductivity at infinite dilution of acetate and hydrogen ions are 349.8 and 40.9 S cm2 mol-1 respectively. Hence the limiting molar conductivity of acetic acid would be written as:
Λ0acetic acid = λ0(H+) + λ0(CH3COO-)
= 40.9 + 349.8
= 390.7 cm2 mol-1
Now, since we are given that the molar conductivity of acetic acid is Λm = 39.05 S cm2 mol-1
Therefore, substituting the values in equation (1), we get:
Degree of dissociation of acetic acid = α = Λm/ Λ0 = `39.05/390.7 = 0.0999 " ≅ " 0.1`
APPEARS IN
संबंधित प्रश्न
The molar conductivity of cation and anion of salt BA are 180 and 220 mhos respectively. The molar conductivity of salt BA at infinite dilution is_____________ .
(a) 90 mhos.cm2
(b) 110 mhos.cm2.mol-1
(c) 200 mhos.cm2.mol-1
(d) 400 mhos.cm2.mol-1
The conductivity of 0.02M AgNO3 at 25°C is 2.428 x 10-3 Ω-1 cm-1. What is its molar
conductivity?
A steady current of 2 amperes was passed through two electrolytic cells X and Y connected in series containing electrolytes FeSO4and ZnSO4 until 2.8g of Fe deposited at the cathode of cell X. How long did the current flow? Calculate the mass of Zn deposited at the cathode of cell Y.
(Molar mass: Fe=56g mol-1,Zn=65.3g mol-1,1F=96500C mol-1)
In the plot of molar conductivity (∧m) vs square root of concentration (c1/2) following curves are obtained for two electrolytes A and B : 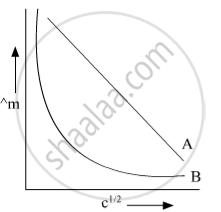
Answer the following:
(i) predict the nature of electrolytes A and B.
(ii) What happens on the extrapolation of ∧m to concentration approaching for electrolytes A and B?
Write the cell reaction of a lead storage battery when it is discharged. How does the density of the electrolyte change when the battery is discharged?
Which of the following halogen acids is the strongest reducing agent?
The molar conductivity of 0.007 M acetic acid is 20 S cm2 mol−1. What is the dissociation constant of acetic acid? Choose the correct option.
`[(Λ_("H"^+)^ο = 350 "S" "cm"^2 "mol"^-1), (Λ_("CH"_3"COO"^-)^ο = 50 "S" "cm"^2 "mol"^-1)]`
The solubility of Co2[Fe(CN)6] in water at 25°C from the following data:
Conductivity of saturated solution of Co2[Fe(CN)6] = 2.06 × 10−6 ohm−1 cm−1 and that of water = 4.1 × 10−7 ohm−1 cm−1. The ionic molar conductivities of Co2+ and [Fe(CN)6]4− are 86 and 444 ohm−1 cm2 mol−1 respectively, is ______ × 10−6 mol/L.
The following questions are case-based questions. Read the passage carefully and answer the questions that follow:
| Rahul set up an experiment to find the resistance of aqueous KCl solution for different concentrations at 298 K using a conductivity cell connected to a Wheatstone bridge. He fed the Wheatstone bridge with a.c. power in the audio frequency range 550 to 5000 cycles per second. Once the resistance was calculated from the null point, he also calculated the conductivity K and molar conductivity ∧m and recorded his readings in tabular form. |
| S. No. | Conc. (M) |
k S cm−1 | ∧m S cm2 mol−1 |
| 1. | 1.00 | 111.3 × 10−3 | 111.3 |
| 2. | 0.10 | 12.9 × 10−3 | 129.0 |
| 3. | 0.01 | 1.41 × 10−3 | 141.0 |
Answer the following questions:
(a) Why does conductivity decrease with dilution? (1)
(b) If `∧_"m"^0` of KCl is 150.0 S cm2 mol−1, calculate the degree of dissociation of 0.01 M KCI. (1)
(c) If Rahul had used HCl instead of KCl then would you expect the ∧m values to be more or less than those per KCl for a given concentration? Justify. (2)
OR
(c) Amit a classmate of Rahul repeated the same experiment with CH3COOH solution instead of KCl solution. Give one point that would be similar and one that would be different in his observations as compared to Rahul. (2)
The specific conductance of 2.5 × 10-4 M formic acid is 5.25 × 10-5 ohm-1 cm-1. Calculate its molar conductivity and degree of dissociation.
Given `λ°_("H"^+)` = 349.5 ohm-1 cm2 mol-1 and
`λ°_("HCOO"^-) = 50.5 " ohm"^-1 "cm"^2 "mol"^-1`
