Advertisements
Advertisements
प्रश्न
Compounds such as alcohols and glucose also contain hydrogen but are not categorized as acids. Describe an activity to prove it.
उत्तर
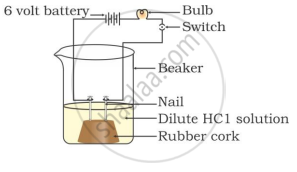
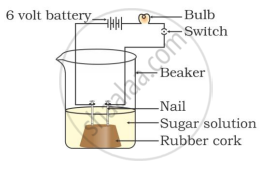
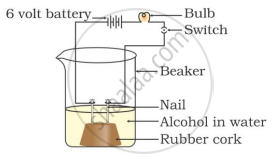
Two nails are fitted on a cork and kept in a 100-mL beaker. The nails are then connected to the two terminals of a 6-volt battery through a bulb and a switch. Some dilute HCl is poured into the beaker, and the current is switched on. The same experiment is then performed with a glucose solution and an alcohol solution.
Observations:
It will be observed that the bulb glows in the HCl solution and does not glow in the glucose solution.
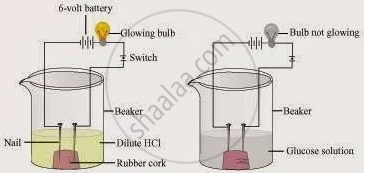
Result:
HCl dissociates into H+ and Cl− ions. These ions conduct electricity in the solution, resulting in the glowing of the bulb. On the other hand, the glucose solution does not dissociate into ions. Therefore, it does not conduct electricity.
Conclusion:
From this activity, it can be concluded that all acids contain hydrogen, but not all compounds containing hydrogen are acids.
That is why, though alcohols and glucose contain hydrogen, they are not categorised as acids.
APPEARS IN
संबंधित प्रश्न
Why does distilled water not conduct electricity, whereas rain water does?
Lime water turns milky when __________ gas is passed through it.
(a) H2
(b) CO
(c) CO2
(d) SO2
What colours do the following indicators turn when added to an acid (such as hydrochloric acid)?
methyl orange
Fill in the blank in the following sentences:
Acids produce.......................... ions on dissolving in water.
What happens when dilute hydrochloric acid is added to sodium carbonate? Write a balanced chemical equation of the reaction involved.
Which gas is liberated when dilute hydrochloric acid reacts with sodium carbonate?
What happens when carbon dioxide gas is passed through lime water for a short time?
Write equations of the reactions involved.
You have been provided with three test-bubes. One of these test-tubes contains distilled water and the other two contain an acidic and a basic solution respectively. If you are given only blue litmus paper, how will you identify the contents of each test-tube?
What is common in all the water soluble bases (or alkalis)?
What is meant by strong bases and weak bases? Classify the following into strong bases and weak bases:
NH4OH, Ca(OH)2, NaOH, KOH, Mg(OH)2
What happens when carbon dioxide gas is passed through lime water for a considerable time ?
Write equations of the reactions involved.
Answer the following question.
Blue litmus solution is added to two test tubes A and B containing dilute HCl and NaOH solution respectively. In which test tube a colour change will be observed? State the colour change and give its reason.
In the experimental set-up to show that "the germinating seeds give out carbon dioxide", answer the following questions:
(i) Why do we keep the conical flask airtight?
(ii) Name the substance kept in the small test tube inside the conical flask. Write its role.
(iii) Why does water rise in the delivery tube?
In which of the following setups would the bulb glow?
Which of the following is(are) true when HCl (g) is passed through water?
- It does not ionise in the solution as it is a covalent compound.
- It ionises in the solution
- It gives both hydrogen and hydroxyl ions in the solution
- It forms hydronium ion in the solution due to the combination of hydrogen ion with water molecule
Identify the correct representation of reaction occurring during chloralkali process
Salt A commonly used in bakery products on heating gets converted into another salt B which itself is used for the removal of hardness of water and a gas C is evolved. The gas C when passed through lime water, turns it milky. Identify A, B and C.
- A compound 'A' with a molecular formula of \[\ce{C2H4O2}\] reacts with a base to give salt and water. Identify 'A', state its nature and the name of the functional group it possesses. Write chemical equation for the reaction involved.
- When the above stated compound 'A' reacts with another compound 'B' having molecular formula \[\ce{C2H6O}\] in the presence of an acid, a sweet smelling compound is 'C' formed.
- Identify 'B' and 'C'.
- State the role of acid in this reaction.
- Write chemical equation for the reaction involved.