Advertisements
Advertisements
प्रश्न
Copper sulphate solution is electrolyzed using copper electrodes. Study the diagram given alongside and answer the questions that follow.
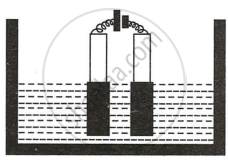
- Which electrode to your left or right is known as the oxidizing electrode and why?
- Write the equation representing the reaction that occurs.
- State two appropriate observations for the above electrolysis reactions.
उत्तर
- The electrode on the left (anode) side donates electrons and hence is the oxidizing electrode.
- \[\ce{Cu - 2e- -> Cu^{2+}}\]
- The anode size steadily lowers while the cathode size gradually increases. However, the colour of the copper sulphate solution does not change.
APPEARS IN
संबंधित प्रश्न
Identify the substance underlined, in the following case
The particles present in a liquid such as kerosene, that is a nonelectrolyte.
State the observations at the anode and at the cathode during the electrolysis of fused lead bromide using graphite electrodes
Give appropriate scientific reasons for During electrolysis of molten lead bromide, graphite anode is preferred to other electrodes.
Define the following term:
Non-electrolyte
What particles are present in pure lead bromide?
A metal article is to be electroplated with silver. The electrolyte selected is sodium argentocyanide.
- What kind of salt is sodium argentocyanide?
- Why is it preferred to silver nitrate as an electrolyte?
- State one condition to ensure that the deposit is smooth, firm and long lasting.
- Write the reaction taking place at the cathode.
- Write the reaction taking place at the anode.
Three different electrolytic cells, A, B and C are connected in separate circuits. Electrolytic cell A contains a sodium chloride solution. When the circuit is completed, a bulb in the circuit glows brightly. Electrolytic cell B contains an acetic acid solution and in this case, the bulb in the circuit glows dimly. The electrolytic cell C contains a sugar solution and the bulb does not glow. Give a reason for each of these observations.
Identify the substance underlined in each of the following case :
he electrolyte used for electroplating an article with silver.
What kind of particles will be found in a liquid compound which is a non-electrolyte?
Classify the following substance:
Dilute hydrochloric acid
