Advertisements
Advertisements
प्रश्न
Define the following term : Brownian movement
उत्तर
The continuous random zig-zag motion of colloidal particles in the colloidal solution, when observed under an ultramicroscope, is called Brownian movement/motion.
संबंधित प्रश्न
Define the following term:
Zeta potential
Explain what is observed An electrolyte, NaCl is added to hydrated ferric oxide sol.
Explain the term Electrophoresis
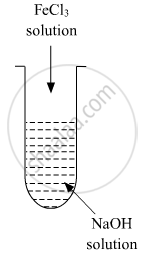
A colloidal sol is prepared by the given method in the figure. What is the charge on hydrated ferric oxide colloidal particles formed in the test tube? How is the sol represented?
Which of the following will show Tyndall effect?
What causes brownian motion in colloidal dispersion?
Assertion: Colloidal solutions do not show brownian motion.
Reason: Brownian motion is responsible for stability of sols.
Gold number is associated with:-
What is Electrophoresis?
The conditions given below are in the context of observing Tyndall effect in colloidal solutions:
- The diameter of the colloidal particles is comparable to the wavelength of light used.
- The diameter of the colloidal particles is much smaller than the wavelength of light used.
- The diameter of the colloidal particles is much larger than the wavelength of light used.
- The refractive indices of the dispersed phase and the dispersion medium are comparable.
- The dispersed phase has a very different refractive index from the dispersion medium.
Choose the most appropriate conditions from the options given below:
