Advertisements
Advertisements
प्रश्न
The rate constant of a first order reaction increases from 2 × 10−2 to 4 × 10−2 when the temperature changes from 300 K to 310 K. Calculate the energy of activation (Ea).
(log 2 = 0.301, log 3 = 0.4771, log 4 = 0.6021)
उत्तर
According to the Arrhenius equation,
k=Ae(−Ea/RT)
From this, we get
`"log"k_2/k_1=E_a/(2.303R)((T_2-T_1)/(T_1T_2))`
We are given that
Initial temperature, T1=300 K
Final temperature, T2=310 K
Rate constant at initial temperature, k1=2×10−2
Rate constant at final temperature, k2=4×10−2
Gas constant, R=8.314 J K−1 mol−1
Substituting the values, we get
`"log"((4xx10^(-2))/(2xx10^(-2)))=E_a/(2.303xx8.314)((310-300)/(300xx310))`
`therefore " activation energy of the reaction, "E_a=(log2xx2.303xx8.314xx300xx310)/10`
`=535985.94" J mol"^(-1)`
`=535.98" kJ mol"^(-1)`
संबंधित प्रश्न
The rate constant for the decomposition of hydrocarbons is 2.418 × 10−5 s−1 at 546 K. If the energy of activation is 179.9 kJ/mol, what will be the value of pre-exponential factor?
What is the effect of adding a catalyst on Activation energy (Ea)
Write a condition under which a bimolecular reaction is kinetically first order. Give an example of such a reaction. (Given : log2 = 0.3010,log 3 = 0.4771, log5 = 0.6990).
Predict the main product of the following reactions: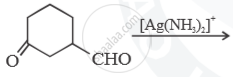
During decomposition of an activated complex:
(i) energy is always released
(ii) energy is always absorbed
(iii) energy does not change
(iv) reactants may be formed
The reaction between \[\ce{H2(g)}\] and \[\ce{O2(g)}\] is highly feasible yet allowing the gases to stand at room temperature in the same vessel does not lead to the formation of water. Explain.
For an endothermic reaction energy of activation is Ea and enthalpy of reaction ΔH (both of there in KJ moI–1) minimum value of Ea will be
In respect of the eqn k = \[\ce{Ae^{{-E_a}/{RT}}}\] in chemical kinetics, which one of the following statement is correct?
The activation energy in a chemical reaction is defined as ______.
The slope of Arrhenius Plot `("In" "k" "v"//"s" 1/"T")` of first-order reaction is −5 × 103 K. The value of Ea of the reaction is. Choose the correct option for your answer. [Given R = 8.314 JK−1mol−1]
