Advertisements
Advertisements
प्रश्न
The rate constant for the decomposition of hydrocarbons is 2.418 × 10−5 s−1 at 546 K. If the energy of activation is 179.9 kJ/mol, what will be the value of pre-exponential factor?
उत्तर
k = 2.418 × 10−5 s−1
T = 546 K
Ea = 179.9 kJ mol−1
According to the Arrhenius equation,
log A = log k + `"E"_"a"/(2.303 "RT")`
= log (2.418 × 10−5) + `179.9/(2.303 xx 8.314 xx 10^(-3) xx 546)`
= (−5 + 0.3834) + 17.2081
= 12.5924 s−1
or, A = Antilog (12.5924) s−1 = 3.902 × 1012 s−1
APPEARS IN
संबंधित प्रश्न
(b) Rate constant ‘k’ of a reaction varies with temperature ‘T’ according to the equation:
`logk=logA-E_a/2.303R(1/T)`
Where Ea is the activation energy. When a graph is plotted for `logk Vs. 1/T` a straight line with a slope of −4250 K is obtained. Calculate ‘Ea’ for the reaction.(R = 8.314 JK−1 mol−1)
What will be the effect of temperature on rate constant?
Consider a certain reaction \[\ce{A -> Products}\] with k = 2.0 × 10−2 s−1. Calculate the concentration of A remaining after 100 s if the initial concentration of A is 1.0 mol L−1.
The decomposition of hydrocarbon follows the equation k = `(4.5 xx 10^11 "s"^-1) "e"^(-28000 "K"//"T")`
Calculate Ea.
A first-order reaction is 50% completed in 40 minutes at 300 K and in 20 minutes at 320 K. Calculate the activation energy of the reaction. (Given : log 2 = 0·3010, log 4 = 0·6021, R = 8·314 JK–1 mol–1)
Write a condition under which a bimolecular reaction is kinetically first order. Give an example of such a reaction. (Given : log2 = 0.3010,log 3 = 0.4771, log5 = 0.6990).
Consider figure and mark the correct option.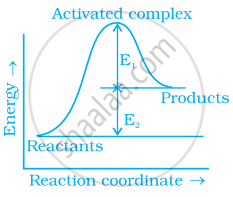
Mark the incorrect statements:
(i) Catalyst provides an alternative pathway to reaction mechanism.
(ii) Catalyst raises the activation energy.
(iii) Catalyst lowers the activation energy.
(iv) Catalyst alters enthalpy change of the reaction.
The reaction between \[\ce{H2(g)}\] and \[\ce{O2(g)}\] is highly feasible yet allowing the gases to stand at room temperature in the same vessel does not lead to the formation of water. Explain.
Thermodynamic feasibility of the reaction alone cannot decide the rate of the reaction. Explain with the help of one example.
Match the statements given in Column I and Column II
| Column I | Column I | |
| (i) | Catalyst alters the rate of reaction | (a) cannot be fraction or zero |
| (ii) | Molecularity | (b) proper orientation is not there always |
| (iii) | Second half life of first order reaction | (c) by lowering the activation energy |
| (iv) | `e^((-E_a)/(RT)` | (d) is same as the first |
| (v) | Energetically favourable reactions (e) total probability is one are sometimes slow | (e) total probability is one |
| (vi) | Area under the Maxwell Boltzman curve is constant | (f) refers to the fraction of molecules with energy equal to or greater than activation energy |
The activation energy in a chemical reaction is defined as ______.
Arrhenius equation can be represented graphically as follows:

The (i) intercept and (ii) slope of the graph are:
Explain how and why will the rate of reaction for a given reaction be affected when the temperature at which the reaction was taking place is decreased.
The activation energy of one of the reactions in a biochemical process is 532611 J mol–1. When the temperature falls from 310 K to 300 K, the change in rate constant observed is k300 = x × 10–3 k310. The value of x is ______.
[Given: ln 10 = 2.3, R = 8.3 J K–1 mol–1]
The decomposition of N2O into N2 and O2 in the presence of gaseous argon follows second-order kinetics, with k = (5.0 × 1011 L mol−1 s−1) `"e"^(-(29000 "K")/"T")`. Arrhenius parameters are ______ kJ mol−1.
A first-order reaction is 50% complete in 30 minutes at 300 K and in 10 minutes at 320 K. Calculate activation energy (Ea) for the reaction. [R = 8.314 J K−1 mol−1]
[Given: log 2 = 0.3010, log 3 = 0.4771, log 4 = 0.6021]
