Advertisements
Advertisements
प्रश्न
Give reasons for the following:
(CH3)3C–O–CH3 on reaction with HI gives (CH3)3C–I and CH3–OH as the main products and not (CH3)3C–OH and CH3–I.
उत्तर १
Usually, iodide, being a big nucleophile, attacks on the group with low steric hindrance and the reaction proceeds by SN2 mechanism.
However, in this case, methanol, on leaving generates a tertiary carbocation, which is more stable. Hence, this reaction proceeds by SN1 mechanism and therefore, we get (CH3)3C-I and CH3-OH as the major products.

उत्तर २
CH3)3-C–O–CH3 is an ether with two different alkyl groups, of which (CH3)3-C-, a tertiary alkyl group, on reaction with hydrogen halide (HI) forms a tertiary halide. This occurs as the reaction is an SN1 reaction. The reaction involves the formation of a stable carbocation. If the ether has a primary alkyl group, then the reaction follows the SN2 mechanism.
APPEARS IN
संबंधित प्रश्न
In the following pair of halogen compounds, which compound undergoes a faster SN1 reaction?
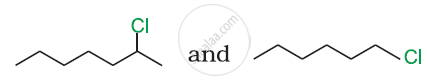
How will you bring about the following conversion?
Toluene to benzyl alcohol
Arrange the compounds of the following set in order of reactivity towards SN2 displacement:
1-Bromobutane, 1-Bromo-2, 2-dimethylpropane, 1-Bromo-2-methylbutane, 1-Bromo-3-methylbutane
Which would undergo SN2 reaction faster in the following pair and why ?
CH3 – CH2 – Br and CH3 – CH2 – I
What is the action of the following on ethyl bromide?
moist silver oxide
2-Bromopentane is heated with potassium ethoxide in ethanol. The major product obtained is ____________.
SN1 reaction of alkyl halides lead to ___________.
The increasing order of reactivity towards SN1 mechanism is:
(I) \[\begin{array}{cc}
\ce{CH3-CH-CH2-CH3}\\
|\phantom{........}\\
\ce{CH3}\phantom{.....}
\end{array}\]
(II) CH3CH2CH2Cl
(III) P–CH3O–C6H4–CH2Cl
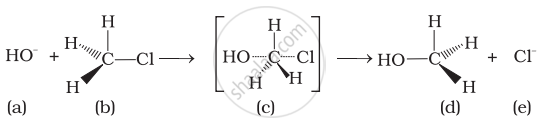
Which of the statements are correct about above reaction?
(i) (a) and (e) both are nucleophiles.
(ii) In (c) carbon atom is sp3 hybridised.
(iii) In (c) carbon atom is sp2 hybridised.
(iv) (a) and (e) both are electrophiles.
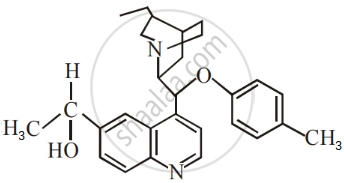
The number of chiral carbons present in the molecule given below is ______.