Advertisements
Advertisements
प्रश्न
Describe the usefulness of water in biosphere and biological systems.
उत्तर
Water is essential for all forms of life. It constitutes around 65% of the human body and 95% of plants. Water plays an important role in the biosphere owing to its high specific heat, thermal conductivity, surface tension, dipole moment, and dielectric constant.
The high heat of vapourization and heat of capacity of water helps in moderating the climate and body temperature of all living beings.
It acts as a carrier of various nutrients required by plants and animals for various metabolic reactions.
APPEARS IN
संबंधित प्रश्न
Among NH3, H2O and HF, which would you expect to have highest magnitude of hydrogen bonding and why?
Why is it said that-
Water is a universal solvent.
State whether the following statement is true or false.
The substance in which a solute dissolves is called a solvent.
Explain the picture in your own words.
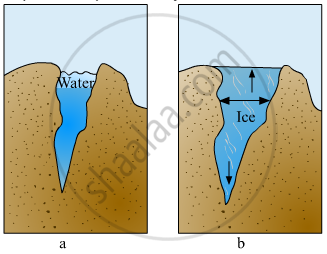
Explain the various properties of water.
Why should a glass bottle completely filled with water never be kept in a freezer?
Define the following.
Boiling point
Which of the following equation depicts reducing nature of \[\ce{H2O2}\]?
Some of the properties of water are described below. Which of them is/are not correct?
(i) Water is known to be a universal solvent.
(ii) Hydrogen bonding is present to a large extent in liquid water.
(iii) There is no hydrogen bonding in the frozen state of water.
(iv) Frozen water is heavier than liquid water.
Give reasons: Lakes freeze from top towards bottom.
Explain why \[\ce{HCl}\] is a gas and \[\ce{HF}\] is a liquid.
Why does water show high boiling point as compared to hydrogen sulphide? Give reasons for your answer.
Write two reactions to explain amphoteric nature of water.
Pure water boils at ______ °C at one atmospheric pressure.
______ has the highest latent heat of fusion.
Water is circulated around the car engine using the ______ pump and the heat is absorbed.
What are the physical properties of pure water?
Which of the following ion is responsible for the temporary hardness of water?
