Advertisements
Advertisements
प्रश्न
Draw the geometrical isomers of complex \[\ce{[Pt(en)2Cl2]^2+}\].
Draw the geometrical isomers of the given complex:
\[\ce{[Pt(en)2Cl2]^2+}\]
उत्तर १
Geometrical isomers of complex\[\ce{[Pt(en)2Cl2]^2+}\]
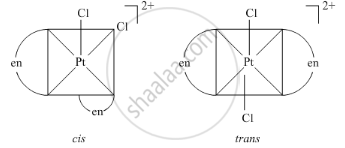
उत्तर २
Geometrical isomers of Dichloridobisethane-1, 2-diammine platinum (iv) ion
 |
 |
संबंधित प्रश्न
Draw the geometrical isomers of complex [Pt(NH3)2Cl2].
Indicate the types of isomerism exhibited by the following complex and draw the structure for this isomer:
[Pt(NH3)(H2O)Cl2]
How many geometrical isomers are possible in the following coordination entity?
[Cr(C2O4)3]3−
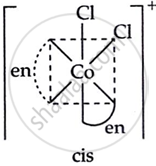
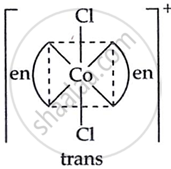
Draw all the isomers (geometrical and optical) of [CoCl2(en)2]+.
Write the structures of optical isomers of the complex ion `[Co(en)_2Cl_2]^+`
What type of structural isomers are [Co(NH3)5 Br] SO4 and [Co(NH3)5 SO4]Br? Give a chemical test to distinguish the isomers.
Identify the optically active compounds from the following:
(i) \[\ce{[Co(en)3]^{3+}}\]
(ii) \[\ce{[trans - [Co(en)2Cl2]^+}\]
(iii) \[\ce{cis - [Co(en)2Cl2]^+}\]
(iv) \[\ce{[Cr(NH3)5Cl]}\]
Which one of the following complexes shows optical isomerism? (en = ethylenediamine)
Indicate the types of isomerism exhibited by the following complexes and draw the structure for isomers:
\[\ce{[Pt(NH3)(H2O)Cl2]}\]
Indicate the types of isomerism exhibited by the following complexes and draw the structure for these isomer:
\[\ce{[Pt(NH3)(H2O)Cl2]}\]
