Advertisements
Advertisements
प्रश्न
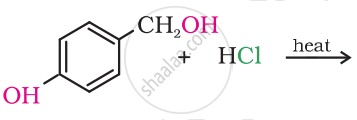
Draw the structure of major monohalo products in the following reaction:

उत्तर
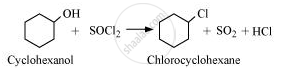
Chlorocyclohexane is the major monohalo product.
APPEARS IN
संबंधित प्रश्न
Draw the structure of major monohalo products in the following reaction:
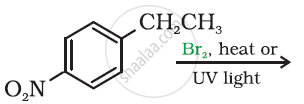
Draw the structure of major monohalo products in the following reaction:
Name the following halide according to the IUPAC system and classify it as an alkyl, allyl, benzoyl (primary, secondary, tertiary), vinyl or aryl halide:
CH3CH=CHC(Br)(CH3)2
When two halogen atoms are attached to the same carbon atom then it is:
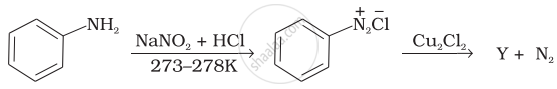
Identify the compound Y in the following reaction.
The position of \[\ce{-Br}\] in the compound in \[\ce{CH3CH = CH(Br)(CH3)2}\] can be classified as ______.
Haloalkanes contain halogen atom (s) attached to the sp3 hybridised carbon atom of an alkyl group. Identify haloalkane from the following compounds.
(i) 2-Bromopentane
(ii) Vinyl chloride (chloroethene)
(iii) 2-chloroacetophenone
(iv) Trichloromethane
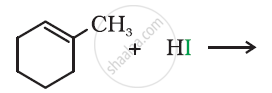
Which of the products will be major product in the reaction given below? Explain.
\[\ce{CH3CH = CH2 + HI -> \underset{(A)}{CH3CH2CH2I} + \underset{(B)}{CH3CHICH3}}\]
Match the items of Column I and Column II.
| Column I | Column II | |
| (i) | SN1 reaction | (a) vic-dibromides |
| (ii) | Chemicals in fire extinguisher | (b) gem-dihalides |
| (iii) | Bromination of alkenes | (c) Racemisation |
| (iv) | Alkylidene halides | (d) Saytzeff rule |
| (v) | Elimination of HX from alkylhalide | (e) Chlorobromocarbons |
Match the structures of compounds given in Column I with the classes of compounds given in Column II.
| Column I | Column II | |
| (i) | \[\begin{array}{cc} \ce{CH3 - CH - CH3}\\ |\phantom{..}\\ \ce{X}\phantom{..} \end{array}\] |
(a) Aryl halide |
| (ii) | \[\ce{CH2 = CH - CH2 - X}\] | (b) Alkyl halide |
| (iii) | 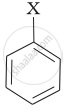 |
(c) Vinyl halide |
| (iv) | \[\ce{CH2 = CH - X}\] | (d) Allyl halide |
Classify the following compound as a primary, secondary and tertiary halide.
1-Bromobut-2-ene
Two isomers (A) and (B) with molar mass 184 g/mol and elemental composition C 52.2%; H 4.9% and Br 42.9% gave benzoic acid and p-bromobenzoic acid, respectively on oxidation with KMnO4. Isomer ‘A’ is optically active and gives a pale yellow precipitate when warmed with alcoholic AgNO3. Isomer ‘A’ and ‘B’ are, respectively:
Name the following halide according to IUPAC system and classify it as alkyl, allyl, benzyl (primary, secondary, tertiary), vinyl or aryl halide:
\[\ce{CH2CH2C(CH3)2CH2I}\]
Name the following halides according to IUPAC system and classify them as alkyl, allyl, benzyl (primary, secondary, tertiary), vinyl or aryl halides:
\[\ce{CH3C(C2H5)2CH2Br}\]
Name the following halides according to IUPAC system and classify them as alkyl, allyl, benzyl (primary, secondary, tertiary), vinyl or aryl halides:
CH3C(Cl)(C2H5 )CH2CH3
Name the following halide according to IUPAC system and classify them as alkyl, allyl, benzyl (primary, secondary, tertiary), vinyl or aryl halides:
\[\ce{CH3C(Cl)(C2H5)CH2CH3}\]
Name the following halide according to IUPAC system and classify them as alkyl, allyl, benzyl (primary, secondary, tertiary), vinyl or aryl halides:
\[\ce{CH3C(C2H5)2CH2Br}\]
Draw the structure of major monohalo products in the following reaction: