Advertisements
Advertisements
प्रश्न
Explain the deviation in ionisation enthalpy of some elements from the general trend by using the given figure.
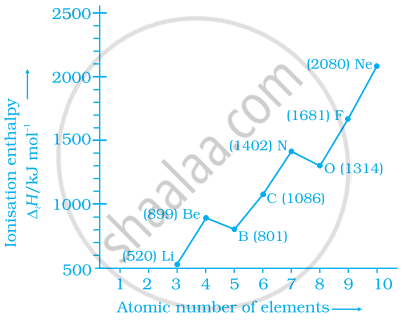
उत्तर
The deviation in the ionization enthalpy of some elements from the general trend can be explained by the points as given below:-
(i) The fully filled and half-filled orbital provide extra stability due to the symmetry.
(ii) The effective nuclear charge.
(iii) The \[\ce{e- - e-}\] repulsion which lead to instability.
APPEARS IN
संबंधित प्रश्न
Among the second period elements the actual ionization enthalpies are in the
order Li < B < Be < C < O < N < F < Ne.
Explain why Be has higher ΔiH than B?
How would you explain the fact that the first ionization enthalpy of sodium is lower than that of magnesium but its second ionization enthalpy is higher than that of magnesium?
The first ionization enthalpy values (in kJmol–1) of group 13 elements are:-
| B | Al | Ga | In | Tl |
| 801 | 577 | 579 | 558 | 589 |
How would you explain this deviation from the general trend?
Would you expect the first ionization enthalpies for two isotopes of the same element to be the same or different? Justify your answer.
Among the elements \[\ce{B, Al, C}\] and \[\ce{Si}\], which element has the highest first ionisation enthalpy?
Arrange the elements \[\ce{N, P, O}\] and \[\ce{S}\] in the order of increasing first ionisation enthalpy. Give reason for the arrangement assigned.
In general, the property (magnitudes only) that shows an opposite trend in comparison to other properties across a period is ______.
For the gaseous reaction, \[\ce{K_{(g)} + F_{(g)} -> K^+_{ (g)} + F^-_{ (g)}}\], ΔH was calculated to be 19 kcal/mol under conditions where the cations and anions were prevented by electrostatic separation from combining with each other. The ionisation energy of K is 4.3 eV. The electron affinity of F is ______. (in eV)
`"A"_0/2` atoms of X(g) are converted into X+(g) by absorbing energy E1. `"A"_0/2` ions of X+(g) are converted into X−(g) with release of energy E2. Hence ionization energy and electron affinity of X(g) are ______.
Which of the following atoms has the highest first ionization energy?
