Advertisements
Advertisements
Question
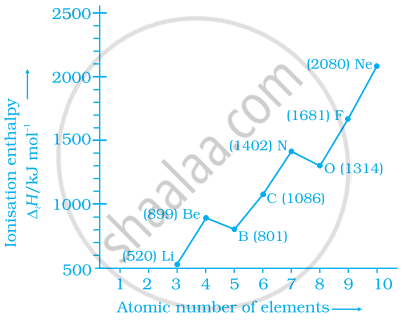
Explain the deviation in ionisation enthalpy of some elements from the general trend by using the given figure.
Solution
The deviation in the ionization enthalpy of some elements from the general trend can be explained by the points as given below:-
(i) The fully filled and half-filled orbital provide extra stability due to the symmetry.
(ii) The effective nuclear charge.
(iii) The \[\ce{e- - e-}\] repulsion which lead to instability.
APPEARS IN
RELATED QUESTIONS
Among the second period elements the actual ionization enthalpies are in the
order Li < B < Be < C < O < N < F < Ne.
Explain why O has lower ΔiH than N and F?
What are the various factors due to which the ionization enthalpy of the main group elements tends to decrease down a group?
Would you expect the first ionization enthalpies for two isotopes of the same element to be the same or different? Justify your answer.
Which one of the following statements is incorrect in relation to ionization enthalpy?
Those elements impart colour to the flame on heating in it, the atoms of which require low energy for the ionisation (i.e., absorb energy in the visible region of spectrum). The elements of which of the following groups will impart colour to the flame?
(i) 2
(ii) 13
(iii) 1
(iv) 17
Nitrogen has positive electron gain enthalpy whereas oxygen has negative. However, oxygen has lower ionisation enthalpy than nitrogen. Explain.
Arrange the elements \[\ce{N, P, O}\] and \[\ce{S}\] in the order of increasing first ionisation enthalpy. Give reason for the arrangement assigned.
Explain the following:
Ionisation enthalpy decrease in a group from top to bottom?
Assertion (A): Generally, ionisation enthalpy increases from left to right in a period.
Reason (R): When successive electrons are added to the orbitals in the same principal quantum level, the shielding effect of inner core of electrons does not increase very much to compensate for the increased attraction of the electron to the nucleus.
Which of the following atoms has the highest first ionization energy?
