Advertisements
Advertisements
Question
Those elements impart colour to the flame on heating in it, the atoms of which require low energy for the ionisation (i.e., absorb energy in the visible region of spectrum). The elements of which of the following groups will impart colour to the flame?
(i) 2
(ii) 13
(iii) 1
(iv) 17
Solution
(i) 2
(iii) 1
Explanation:
The elements of group 1 (alkali metals) and group 2 (alkaline earth metals) have low ionization enthalpies. Therefore, they impart colour to flame.
APPEARS IN
RELATED QUESTIONS
What are the various factors due to which the ionization enthalpy of the main group elements tends to decrease down a group?
Would you expect the first ionization enthalpies for two isotopes of the same element to be the same or different? Justify your answer.
Among the elements \[\ce{B, Al, C}\] and \[\ce{Si}\], which element has the highest first ionisation enthalpy?
Explain the deviation in ionisation enthalpy of some elements from the general trend by using the given figure.
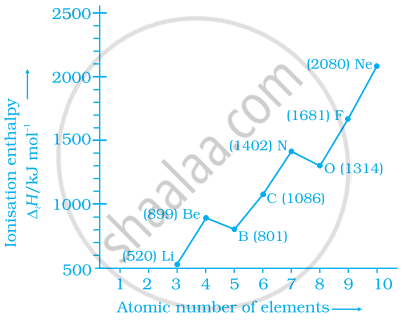
Explain the following:
Ionisation enthalpy decrease in a group from top to bottom?
Assertion (A): Generally, ionisation enthalpy increases from left to right in a period.
Reason (R): When successive electrons are added to the orbitals in the same principal quantum level, the shielding effect of inner core of electrons does not increase very much to compensate for the increased attraction of the electron to the nucleus.
Discuss and compare the trend in ionisation enthalpy of the elements of group1 with those of group17 elements.
Consider the elements Mg, Al, S, P and Si, the correct increasing order of their first ionization enthalpy is ______.
`"A"_0/2` atoms of X(g) are converted into X+(g) by absorbing energy E1. `"A"_0/2` ions of X+(g) are converted into X−(g) with release of energy E2. Hence ionization energy and electron affinity of X(g) are ______.
Which of the following atoms has the highest first ionization energy?
