Advertisements
Online Mock Tests
Chapters
2: Structure of Atom
▶ 3: Classification of Elements and Periodicity in Properties
4: Chemical Bonding and Molecular Structure
5: States of Matter
6: Thermodynamics
7: Equilibrium
8: Redox Reactions
9: Hydrogen
10: The s-block Elements
11: The p-block Elements
12: Organic Chemistry Some Basic Principles and Techniques
13: Hydrocarbons
14: Environmental Chemistry
![NCERT Exemplar solutions for Chemistry [English] Class 11 chapter 3 - Classification of Elements and Periodicity in Properties NCERT Exemplar solutions for Chemistry [English] Class 11 chapter 3 - Classification of Elements and Periodicity in Properties - Shaalaa.com](/images/chemistry-english-class-11_6:5f2b1b2038084cf381bfa42c826a928c.jpg)
Advertisements
Solutions for Chapter 3: Classification of Elements and Periodicity in Properties
Below listed, you can find solutions for Chapter 3 of CBSE NCERT Exemplar for Chemistry [English] Class 11.
NCERT Exemplar solutions for Chemistry [English] Class 11 3 Classification of Elements and Periodicity in Properties Multiple Choice Questions (Type - I) [Pages 27 - 36]
Consider the isoelectronic species, \[\ce{Na+, Mg^{2+}, F–}\] and \[\ce{O2-}\]. The correct order of increasing length of their radii is ______.
\[\ce{F- < O^{2-} < Mg^{2+} < Na+}\]
\[\ce{Mg^{2+} < Na+ < F- < O^{2-}}\]
\[\ce{O^{2-} < F- < Na+ < Mg^{2+}}\]
\[\ce{O^{2-} < F- < Mg^{2+} < Na+}\]
Which of the following is not an actinoid?
Curium (Z = 96)
Californium (Z = 98)
Uranium (Z = 92)
Terbium (Z = 65)
The order of screening effect of electrons of s, p, d and f orbitals of a given shell of an atom on its outer shell electrons is ______.
s > p > d > f
f > d > p > s
p < d < s > f
f > p > s > d
The first ionisation enthalpies of \[\ce{Na, Mg, Al}\] and \[\ce{Si}\] are in the order:
\[\ce{Na < Mg > Al < Si}\]
\[\ce{Na > Mg > Al > Si}\]
\[\ce{Na < Mg < Al < Si}\]
\[\ce{Na > Mg > Al < Si}\]
The electronic configuration of gadolinium (Atomic number 64) is ______.
`[Xe] 4f^3 5d^5 6s^2`
`[Xe] 4f^7 5d^2 6s^1`
`[Xe] 4f^7 5d^1 6s^2`
`[Xe] 4f^8 5d^6 6s^2`
The statement that is not correct for periodic classification of elements is ______.
The properties of elements are periodic function of their atomic numbers.
Non metallic elements are less in number than metallic elements.
For transition elements, the 3d-orbitals are filled with electrons after 3p-orbitals and before 4s-orbitals.
The first ionisation enthalpies of elements generally increase with increase in atomic number as we go along a period.
Among halogens, the correct order of amount of energy released in electron gain (electron gain enthalpy) is ______.
\[\ce{F > Cl > Br > I}\]
\[\ce{F < Cl < Br < I}\]
\[\ce{F < Cl > Br > I}\]
\[\ce{F < Cl < Br < I}\]
The period number in the long form of the periodic table is equal to ______.
magnetic quantum number of any element of the period.
atomic number of any element of the period.
maximum Principal quantum number of any element of the period.
maximum Azimuthal quantum number of any element of the period.
The elements in which electrons are progressively filled in 4f-orbital are called ______.
actinoids
transition elements
lanthanoids
halogens
Which of the following is the correct order of size of the given species?
\[\ce{I > I- > I+}\]
\[\ce{I+ > I- > I}\]
\[\ce{I > I+ > I-}\]
\[\ce{I- > I > I+}\]
The formation of the oxide ion, \[\ce{O2- (g)}\], from oxygen atom requires first an exothermic and then an endothermic step as shown below:
\[\ce{O (g) + e- -> O- (g) ; ∆H^Θ = - 14 kJ mol^{-1}}\]
\[\ce{O- (g) + e- -> O^{2-} (g) ; ∆H^Θ = + 780 kJ mol^{-1}}\]
Thus process of formation of \[\ce{O^{2-}}\] in gas phase is unfavourable even though \[\ce{O^{2-}}\] is isoelectronic with neon. It is due to the fact that,
oxygen is more electronegative.
addition of electron in oxygen results in larger size of the ion.
electron repulsion outweighs the stability gained by achieving noble gas configuration.
\[\ce{O-}\] ion has comparatively smaller size than oxygen atom.
In the modern periodic table, elements are arranged in order of increasing atomic numbers which is related to the electronic configuration. Depending upon the type of orbitals receiving the last electron, the elements in the periodic table have been divided into four blocks, viz, s, p, d and f. The modern periodic table consists of 7 periods and 18 groups. Each period begins with the filling of a new energy shell. In accordance with the Arfbau principle, the seven periods have 2, 8, 8, 18, 18, 32 and 32 elements respectively. The seventh period is still incomplete. To avoid the periodic table being too long, the two series of f-block elements, called lanthanoids and actinoids are placed at the bottom of the main body of the periodic table.
The element with atomic number 57 belongs to ______.
s-block
p-block
d-block
f-block
In the modern periodic table, elements are arranged in order of increasing atomic numbers which is related to the electronic configuration. Depending upon the type of orbitals receiving the last electron, the elements in the periodic table have been divided into four blocks, viz, s, p, d and f. The modern periodic table consists of 7 periods and 18 groups. Each period begins with the filling of a new energy shell. In accordance with the Arfbau principle, the seven periods have 2, 8, 8, 18, 18, 32 and 32 elements respectively. The seventh period is still incomplete. To avoid the periodic table being too long, the two series of f-block elements, called lanthanoids and actinoids are placed at the bottom of the main body of the periodic table.
The last element of the p-block in 6th period is represented by the outermost electronic configuration.
7s27p6
5f146d107s27p0
4f145d106s26p6
4f145d106s26p4
In the modern periodic table, elements are arranged in order of increasing atomic numbers which is related to the electronic configuration. Depending upon the type of orbitals receiving the last electron, the elements in the periodic table have been divided into four blocks, viz, s, p, d and f. The modern periodic table consists of 7 periods and 18 groups. Each period begins with the filling of a new energy shell. In accordance with the Arfbau principle, the seven periods have 2, 8, 8, 18, 18, 32 and 32 elements respectively. The seventh period is still incomplete. To avoid the periodic table being too long, the two series of f-block elements, called lanthanoids and actinoids are placed at the bottom of the main body of the periodic table.
Which of the elements whose atomic numbers are given below, cannot be accommodated in the present set-up of the long form of the periodic table?
107
118
126
102
In the modern periodic table, elements are arranged in order of increasing atomic numbers which is related to the electronic configuration. Depending upon the type of orbitals receiving the last electron, the elements in the periodic table have been divided into four blocks, viz, s, p, d and f. The modern periodic table consists of 7 periods and 18 groups. Each period begins with the filling of a new energy shell. In accordance with the Arfbau principle, the seven periods have 2, 8, 8, 18, 18, 32 and 32 elements respectively. The seventh period is still incomplete. To avoid the periodic table being too long, the two series of f-block elements, called lanthanoids and actinoids are placed at the bottom of the main body of the periodic table.
The electronic configuration of the element which is just above the element with atomic number 43 in the same group is ______.
1s2 2s2 2p6 3s2 3p6 3d5 4s2
1s2 2s2 2p6 3s2 3p2 3d5 4s3 4p6
1s2 2s2 2p6 3s2 3p6 3d6 4s2
1s2 2s2 2p6 3s2 3p6 3d7 4s2
In the modern periodic table, elements are arranged in order of increasing atomic numbers which is related to the electronic configuration. Depending upon the type of orbitals receiving the last electron, the elements in the periodic table have been divided into four blocks, viz, s, p, d and f. The modern periodic table consists of 7 periods and 18 groups. Each period begins with the filling of a new energy shell. In accordance with the Arfbau principle, the seven periods have 2, 8, 8, 18, 18, 32 and 32 elements respectively. The seventh period is still incomplete. To avoid the periodic table being too long, the two series of f-block elements, called lanthanoids and actinoids are placed at the bottom of the main body of the periodic table.
The elements with atomic numbers 35, 53 and 85 are all ______.
noble gases
halogens
heavy metals
light metals
Electronic configurations of four elements A, B, C and D are given below:
(A) 1s22s22p6
(B) 1s22s22p4
(C) 1s22s22p63s1
(D) 1s22s22p5
Which of the following is the correct order of increasing tendency to gain electron:
A < C < B < D
A < B < C < D
D < B < C < A
D < A < B < C
Which of the following elements can show covalency greater than 4?
(i) Be
(ii) P
(iii) S
(iv) B
Those elements impart colour to the flame on heating in it, the atoms of which require low energy for the ionisation (i.e., absorb energy in the visible region of spectrum). The elements of which of the following groups will impart colour to the flame?
(i) 2
(ii) 13
(iii) 1
(iv) 17
Which of the following sequences contain atomic numbers of only representative elements?
(i) 3, 33, 53, 87
(ii) 2, 10, 22, 36
(iii) 7, 17, 25, 37, 48
(iv) 9, 35, 51, 88
Which of the following elements will gain one electron more readily in comparison to other elements of their group?
(i) \[\ce{S (g)}\]
(ii) \[\ce{Na (g)}\]
(iii) \[\ce{O (g)}\]
(iv) \[\ce{Cl (g)}\]
Which of the following statements are correct?
(i) Helium has the highest first ionisation enthalpy in the periodic table.
(ii) Chlorine has less negative electron gain enthalpy than fluorine.
(iii) Mercury and bromine are liquids at room temperature.
(iv) In any period, atomic radius of alkali metal is the highest.
Which of the following sets contain only isoelectronic ions?
(i) \[\ce{Zn^{2+}, Ca^{2+}, Ga^{3+}, Al^{3+}}\]
(ii) \[\ce{K+ , Ca^{2+}, Sc^{3+}, Cl-}\]
(iii) \[\ce{P^{3-}, S^{2-}, Cl- , K+}\]
(iv) \[\ce{Ti^{4+}, Ar, Cr^{3+}, V^{5+}}\]
In which of the following options the order of arrangement does not agree with the variation of the property indicated against it?
(i) \[\ce{Al^{3+} < Mg^{2+} < Na+ < F-}\] (increasing ionic size)
(ii) \[\ce{B < C < N < O}\] (increasing first ionisation enthalpy)
(iii) \[\ce{I < Br < Cl < F}\] (increasing electron gain enthalpy)
(iv) \[\ce{Li < Na < K < Rb}\] (increasing metallic radius)
Which of the following have no unit?
(i) Electronegativity
(ii) Electron gain enthalpy
(iii) Ionisation enthalpy
(iv) Metallic character
Ionic radii vary in
(i) inverse proportion to the effective nuclear charge.
(ii) inverse proportion to the square of effective nuclear charge.
(iii) direct proportion to the screening effect.
(iv) direct proportion to the square of screening effect.
An element belongs to 3rd period and group-13 of the periodic table. Which of the following properties will be shown by the element?
(i) Good conductor of electricity
(ii) Liquid, metallic
(iii) Solid, metallic
(iv) Solid, non metallic
Explain why the electron gain enthalpy of fluorine is less negative than that of chlorine.
All transition elements are d-block elements, but all d-block elements are not transition elements. Explain
Identify the group and valency of the element having atomic number 119. Also predict the outermost electronic configuration and write the general formula of its oxide.
Ionisation enthalpies of elements of second period are given below: Ionisation enthalpy/ k cal mol–1:
520, 899, 801, 1086, 1402, 1314, 1681, 2080.
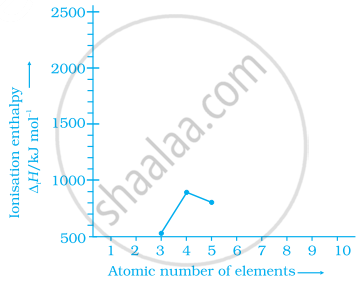
Match the correct enthalpy with the elements and complete the graph given in figure. Also write symbols of elements with their atomic number
Among the elements \[\ce{B, Al, C}\] and \[\ce{Si}\], which element has the highest first ionisation enthalpy?
Among the elements \[\ce{B, Al, C}\] and \[\ce{Si}\], which element has the most metallic character?
Write four characteristic properties of p-block elements.
Choose the correct order of atomic radii of fluorine and neon (in pm) out of the options given below and justify your answer.
72, 160
160, 160
72, 72
160, 72
Illustrate by taking examples of transition elements and non-transition elements that oxidation states of elements are largely based on electronic configuration.
Nitrogen has positive electron gain enthalpy whereas oxygen has negative. However, oxygen has lower ionisation enthalpy than nitrogen. Explain.
First member of each group of representative elements (i.e., s and p-block elements) shows anomalous behaviour. Illustrate with two examples.
p-Block elements form acidic, basic and amphoteric oxides. Explain each property by giving two examples and also write the reactions of these oxides with water.
How would you explain the fact that first ionisation enthalpy of sodium is lower than that of magnesium but its second ionisation enthalpy is higher than that of magnesium?
What do you understand by exothermic reaction and endothermic reaction? Give one example of each type.
Arrange the elements \[\ce{N, P, O}\] and \[\ce{S}\] in the order of increasing first ionisation enthalpy. Give reason for the arrangement assigned.
Arrange the elements \[\ce{N, P, O}\] and \[\ce{S}\] in the order of increasing non metallic character. Give reason for the arrangement assigned.
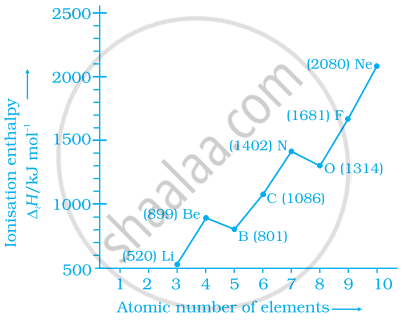
Explain the deviation in ionisation enthalpy of some elements from the general trend by using the given figure.
Explain the following:
Electronegativity of elements increase on moving from left to right in the periodic table.
Explain the following:
Ionisation enthalpy decrease in a group from top to bottom?
How does the metallic and non-metallic character vary on moving from left to right in a period?
The radius of \[\ce{Na+}\] cation is less than that of \[\ce{Na}\] atom. Give reason.
Among alkali metals which element do you expect to be least electronegative and why?
Match the correct atomic radius with the element.
| Element | Atomic radius (pm) |
| \[\ce{Be}\] | 74 |
| \[\ce{C}\] | 88 |
| \[\ce{O}\] | 111 |
| \[\ce{B}\] | 77 |
| \[\ce{N}\] | 66 |
Match the correct ionisation enthalpies and electron gain enthalpies of the following elements.
| Elements | ∆H1 | ∆H2 | ∆egH | |
| (i) Most reactive non-metal | A. | 419 | 3051 | – 48 |
| (ii) Most reactive metal | B. | 1681 | 3374 | – 328 |
| (iii) Least reactive element e | C. | 738 | 1451 | – 40 |
| (iv) Metal forming binary halide | D. | 2372 | 5251 | + 48 |
Electronic configuration of some elements is given in Column I and their electron gain enthalpies are given in Column II. Match the electronic configuration with electron gain enthalpy.
| Column (I) | Column (II) |
| Electronic configuration | Electron gain enthalpy/kJ mol–1 |
| (i) 1s2 2s2 sp6 | (A) – 53 |
| (ii) 1s2 2s2 2p6 3s1 | (B) – 328 |
| (iii) 1s2 2s2 2p5 | (C) – 141 |
| (iv) 1s2 2s2 2p4 | (D) + 48 |
Assertion (A): Generally, ionisation enthalpy increases from left to right in a period.
Reason (R): When successive electrons are added to the orbitals in the same principal quantum level, the shielding effect of inner core of electrons does not increase very much to compensate for the increased attraction of the electron to the nucleus.
Assertion is correct statement and reason is wrong statement.
Assertion and reason both are correct statements and reason is correct explanation of assertion.
Assertion and reason both are wrong statements.
Assertion is wrong statement and reason is correct statement.
Assertion (A): Boron has a smaller first ionisation enthalpy than beryllium.
Reason (R): The penetration of a 2s electron to the nucleus is more than the 2p electron hence 2p electron is more shielded by the inner core of electrons than the 2s electrons.
Assertion and reason both are correct statements but reason is not correct explanation for assertion.
Assertion is correct statement but reason is wrong statement.
Assertion and reason both are correct statements and reason is correct explanation for assertion.
Assertion and reason both are wrong statements.
Assertion (A): Electron gain enthalpy becomes less negative as we go down a group.
Reason (R): Size of the atom increases on going down the group and the added electron would be farther from the nucleus.
Assertion and reason both are correct statements but reason is not correct explanation for assertion.
Assertion and reason both are correct statements and reason is correct explanation for assertion.
Assertion and reason both are wrong statements.
Assertion is wrong statement but reason is correct statement.
Discuss the factors affecting electron gain enthalpy and the trend in its variation in the periodic table.
Define ionisation enthalpy. Discuss the factors affecting ionisation enthalpy of the elements and its trends in the periodic table.
Justify the given statement with suitable examples— “the Properties of the elements are a periodic function of their atomic numbers”.
Write down the outermost electronic configuration of alkali metals. How will you justify their placement in group 1 of the periodic table?
Write the drawbacks in Mendeleev’s periodic table that led to its modification.
In what manner is the long form of periodic table better than Mendeleev’s periodic table? Explain with examples.
Discuss and compare the trend in ionisation enthalpy of the elements of group1 with those of group17 elements.
Solutions for 3: Classification of Elements and Periodicity in Properties
![NCERT Exemplar solutions for Chemistry [English] Class 11 chapter 3 - Classification of Elements and Periodicity in Properties NCERT Exemplar solutions for Chemistry [English] Class 11 chapter 3 - Classification of Elements and Periodicity in Properties - Shaalaa.com](/images/chemistry-english-class-11_6:5f2b1b2038084cf381bfa42c826a928c.jpg)
NCERT Exemplar solutions for Chemistry [English] Class 11 chapter 3 - Classification of Elements and Periodicity in Properties
Shaalaa.com has the CBSE Mathematics Chemistry [English] Class 11 CBSE solutions in a manner that help students grasp basic concepts better and faster. The detailed, step-by-step solutions will help you understand the concepts better and clarify any confusion. NCERT Exemplar solutions for Mathematics Chemistry [English] Class 11 CBSE 3 (Classification of Elements and Periodicity in Properties) include all questions with answers and detailed explanations. This will clear students' doubts about questions and improve their application skills while preparing for board exams.
Further, we at Shaalaa.com provide such solutions so students can prepare for written exams. NCERT Exemplar textbook solutions can be a core help for self-study and provide excellent self-help guidance for students.
Concepts covered in Chemistry [English] Class 11 chapter 3 Classification of Elements and Periodicity in Properties are Significance of Classification of Elements, Genesis of Periodic Classification, Modern Periodic Law and the Present Form of the Periodic Table, Nomenclature of Elements with Atomic Number Greater than 100, Periodic Table and Electronic Configuration, The s-Block Elements, The p-Block Elements, The d-Block Elements (Transition Elements), The f-Block Elements (Inner-transition Elements), Metals, Non-metals and Metalloids, Ionic Radius, Ionization Enthalpy or Ionization Energy (IE) or Ionization Potential (IP), Electron Gain Enthalpy, Electronegativity, Periodicity of Valence or Oxidation States, Anomalous Properties of Second Period Elements, Periodic Trends and Chemical Reactivity, Atomic Radius Or Atomic Size, Classification of Elements and Periodicity in Properties Numericals.
Using NCERT Exemplar Chemistry [English] Class 11 solutions Classification of Elements and Periodicity in Properties exercise by students is an easy way to prepare for the exams, as they involve solutions arranged chapter-wise and also page-wise. The questions involved in NCERT Exemplar Solutions are essential questions that can be asked in the final exam. Maximum CBSE Chemistry [English] Class 11 students prefer NCERT Exemplar Textbook Solutions to score more in exams.
Get the free view of Chapter 3, Classification of Elements and Periodicity in Properties Chemistry [English] Class 11 additional questions for Mathematics Chemistry [English] Class 11 CBSE, and you can use Shaalaa.com to keep it handy for your exam preparation.
