Advertisements
Advertisements
प्रश्न
Explain the non-linear shape of \[\ce{H2S}\] and non-planar shape of \[\ce{PCl3}\] using valence shell electron pair repulsion theory.
उत्तर
The Lewis structure of \[\ce{H2S}\] is:

S-tom is surrounded by four electron pairs (two bonded and two lone pairs). These four electron pairs adopt tetrahedral arrangement. The presence of two lone pairs brings distortion in the molecule on account of repulsion with bonded pairs of electrons. Thus, the shape of \[\ce{H2S}\] molecule is V-shaped and not linear.
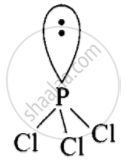
The Lewis structure of \[\ce{PCl3}\] is:
\[\begin{array}{cc}
\ce{Cl - \overset{\bullet\bullet}{P} - Cl}\\
|\phantom{.}\\
\ce{Cl}\phantom{}
\end{array}\]
P-tom is surrounded by four electron pairs (3 bonded and one lone pair). These four pairs adopt a tetrahedral geometry. Due to the presence of lone pair, \[\ce{PCl3}\] has a distorted tetrahedral geometry. Thus, it is pyramidal in shape and not non-planar shape.
APPEARS IN
संबंधित प्रश्न
Select and write the most appropriate alternatives from the given choices.
Valence Shell Electron Pair repulsion (VSEPR) theory is used to predict which of the following:
According to VSEPR theory, the repulsion between different parts of electrons obey the order.
Explain VSEPR theory. Applying this theory to predict the shapes of IF7 and SF6.
The H-N-H bond angle in NH3 molecule is ____________.
Select the INCORRECT match.
Elements \[\ce{X, Y}\] and \[\ce{Z}\] have 4, 5 and 7 valence electrons respectively. Write the molecular formula of the compounds formed by these elements individually with hydrogen.
Which of the possible molecule/species is having maximum values for dipole moment. (where "A" is the central atom)?
Consider the species CH4, `"NH"_4^+` and `"BH"_4^-`. Choose the correct option with respect to these species.
Number of lone pair(s) of electrons on central atom and the shape of BrF3 molecule respectively are ______.
The number of lone pairs of electrons on the central I atom in `"I"_3^-` is ______.
