Advertisements
Advertisements
प्रश्न
Explain the term redox reaction with an example involving the reaction of hydrogen sulphide with chlorine.
उत्तर
Redox Reaction: “The reaction in which oxidation and reduction take place simultaneously is called Redox Reaction.”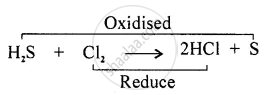
Removal of hydrogen is oxidation. Here `"H"_2"S"` is oxidised to sulphur whereas the addition of hydrogen is reduction.
Here chlorine is being reduced to hydrogen chloride.
APPEARS IN
संबंधित प्रश्न
What are anti-oxidants? Why are they added to fat and oil containing foods?
What is an oxidation reaction? Identify in the following reaction (i) the substance oxidised, and (ii) the substance reduced:
ZnO + C → Zn + CO
Explain oxidation in terms of gain or loss of oxygen with one example.
Identify the substances that are oxidised and the substances that are reduced in the following reaction.
\[\ce{CuO(s) + H2(g) -> Cu(s) + H2O (l)}\]
The electron releasing tendency of zinc is ______ than that of copper.
The colours of aqueous solutions of CuSO4 and FeSO4 as observed in the laboratory are:
(A) pale green and light blue respectively
(B) light blue and dark green respectively
(C) dark blue and dark green respectively
(D) dark blue and pale green respectively
With reference to oxidation & reduction reaction – complete the statement given by filling in the blanks with only the words (a) Addition (b) Removal.
‘Oxidation is a chemical reaction involving ____ of oxygen to a substance or ____ of hydrogen from a substance. Reduction on the otherhand involves ____ of hydrogen to a substance or ____ of oxygen from a substance.
Explain the following reaction with their balanced chemical equation.
Hydrogen sulphide reacts with sulphur dioxide.
In the reaction of iron with copper sulphate solution:
CuSO4 + Fe → Cu + FeSO4
Which option in the given table correctly represents the substance oxidized and the reducing agent?
| OPTION | Substance Oxidized | Reducing Agent |
| A | Fe | Fe |
| B | Fe | FeSO4 |
| C | Cu | Fe |
| D | CuSO4 | Fe |
Which among the following are physical or chemical change?
Heating of an iron rod to red hot
