Advertisements
Advertisements
Question
Explain the term redox reaction with an example involving the reaction of hydrogen sulphide with chlorine.
Solution
Redox Reaction: “The reaction in which oxidation and reduction take place simultaneously is called Redox Reaction.”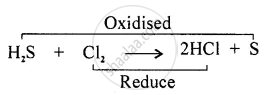
Removal of hydrogen is oxidation. Here `"H"_2"S"` is oxidised to sulphur whereas the addition of hydrogen is reduction.
Here chlorine is being reduced to hydrogen chloride.
APPEARS IN
RELATED QUESTIONS
The chemical formula for rust is.............. .
Define corrosion.
Give an example of an oxidation reaction.
Identify the following reactions as oxidation or reduction
- Na → Na+ + e
- Fe3+ + 2 e- → Fe+
Identify the oxidising agent (oxidant) in the following reactions
`2"Mg" + "O"_2 -> 2"MgO"`
Mention some oxidation reactions that occur in daily life.
______ is a metal that has a high resistance to corrosion.
Define oxidation number.
Answer the questions based on the equation below:
\[\ce{CH3 - CH2 - OH ->[{[O]}][K2Cr2O7/H2SO4] CH3 - COOH}\]
- What type of reaction is it?
- What is the role of the chemical written on the arrow?
- Give one example of one such type of chemical as in answer (b).
The oxidizing agent in the equation \[\ce{S + 2H2SO4 -> 3SO2 + 2H2O}\] is ______.
