Advertisements
Advertisements
प्रश्न
Give a balanced equation for –
A displacement reaction involving a metal above hydrogen in the activity series with copper [II] sulphate solution
उत्तर
Displacement reaction
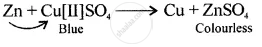
[more reactive displaces less reactive Cu]
APPEARS IN
संबंधित प्रश्न
Reaction of iron nails with copper sulphate solution is an example of
(a) Combination reaction
(b) Decomposition reaction
(c) Displacement reaction
(d) Double displacement reaction
The reddish brown deposit formed on iron nails kept in a solution of copper sulphate is
- Cu2O
- Cu
- CuO
- CuS
\[\ce{Fe2O3 + 2Al -> Al2O3 + 2Fe}\]
The above reaction is an example of a ______.
In the refining of silver, the recovery of silver from silver nitrate solution involved displacement by copper metal. Write down the reaction involved.
State two characteristics of the chemical reaction which takes place when dilute sulphuric acid is poured over zinc granules.
Explain the following type of chemical reaction, giving two examples for it:
Displacement reaction
i) Observe the following reaction and answer the following questions.
`CuSO_4 (aq) + Fe (s) → FeSO_4(aq) + Cu (s)`
a) Identify and write the type of chemical reaction.
b) Write the definition of above reaction.
Give reasons for the following:
The reaction of iron (III) oxide [Fe2O3] with heated aluminum is used to join cracked machine parts.
Which of the following is a displacement reaction?
Complete the given chemical equation:
\[\ce{Zn(s) + CuSO4(aq) -> \underline{}\underline{}\underline{}\underline{}\underline{} + \underline{}\underline{}\underline{}\underline{}\underline{}}\]
Name the type of reaction.
