Advertisements
Advertisements
प्रश्न
Give an example for each of the following statement
A compound in which two Covalent bonds are formed.
उत्तर
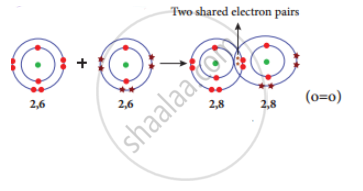
Formation of oxygen molecule (O2): Each oxygen atom has six valence electrons (2, 6). These two atoms achieve a stable electronic configuration (octet) by sharing two pair of electrons. Hence a double bond is formed in between the two atoms.
Formation of covalent bond in oxygen molecule.
APPEARS IN
संबंधित प्रश्न
State one major difference between covalent and ionic bonds and give one example each of covalent and ionic compounds.
The number of isomers formed by the hydrocarbon with molecular formula C5H12 is:
(a) 2
(b) 5
(c) 3
(d) 4
What are the conditions necessary for the formation of covalent molecules?
Explain the Structural isomerism term with example.
Explain the following term with example.
Unsaturated hydrocarbon
Draw the electron dot diagram and structure of :
methane
Potassium chloride is an electrovalent compound, while hydrogen chloride is a covalent compound, But, both conduct electricity in their aqueous solutions. Explain.
The number of electrons in the valence shell of a carbon atom is 4.
Molecular reactions are ______ in the covalent compound.
State the reasons, why carbon cannot
- Lose four electrons to form C4+ cation and
- Gain four electrons to form C4- anion.
How does carbon overcome this problem to form compounds?
