Advertisements
Advertisements
प्रश्न
Give one equation to show the following properties of sulphuric acid:
Dehydrating property
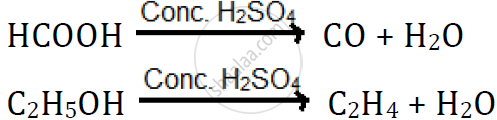
उत्तर
Dehydrating property of sulphuric acid:
H2SO4 has a great affinity for water, and therefore, it acts as a dehydrating agent.
APPEARS IN
संबंधित प्रश्न
Give one equation to show the following properties of sulphuric acid:
Acidic nature
Give reason for the following:
Ammonia gas cannot be dried by passing through concentrated sulphuric acid.
How are the following conversion brought about? Give equation and condition:
Dilute sulphuric acid to hydrogen.
How are the following conversion brought about? Give equation and condition:
Sodium chloride to hydrogen chloride.
Describe the reaction that show
Concentrated sulphuric acid is a non-volatile acid.
Give main difference between drying agent and dehydrating agent.
Name the gas evolved in following case:
The gas produced by the action of concentrated sulphuric acid on sodium chloride.
Give balanced chemical equation for the action of sulphuric acid of the following:
Potassium hydrogen carbonate
An acid obtained from concentrated nitric acid on reaction with Sulphur ______.
The salt is formed when concentrated sulphuric acid reacts with KNO3 above 200°C.
