Advertisements
Advertisements
Question
Give one equation to show the following properties of sulphuric acid:
Dehydrating property
Solution
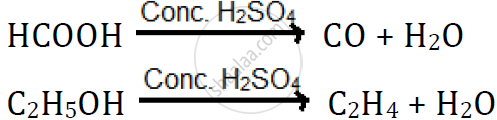
Dehydrating property of sulphuric acid:
H2SO4 has a great affinity for water, and therefore, it acts as a dehydrating agent.
APPEARS IN
RELATED QUESTIONS
Which property of sulphuric acid is shown by the reaction of the concentrated sulphuric acid with Carbon?
Define the following term : Hygroscopic substance
Name the following:
Products obtained by treating zinc with dilute sulphuric acid.
Name the Following:
The property used to prepare HCl and HNO3 from H2SO4.
Give reason for the following:
Concentrated sulphuric acid should not be added to oxalic acid or formic acid in the open laboratory.
Write balanced equation for the reaction of dilute sulphuric acid with the following:
Copper carbonate
State your observation for the following case :
Paper soaked in potassium permanganate solution is introduced into a gas jar of sulphur dioxide.
Write the observation for the following:
Decomposition of bicarbonates by dil. H2SO4
\[\ce{2NaHCO3 + H2SO4 -> Na2SO4 + 2H2O + 2CO2}\]
Distinguish between the following pair of compounds using the test given with brackets:
Dilute sulphuric acid and dilute hydrochloric acid (using barium chloride solution)
Give balanced chemical equation to prepare the following salt:
Lead sulphate from lead carbonate
