Advertisements
Advertisements
प्रश्न
Give one example, state what are covalent compounds?
उत्तर
Covalent compounds are compounds that are formed by the sharing of electrons between two or more atoms.
Example: Hydrogen (H2) is a covalent compound, where each hydrogen atom shares one electron to form a stable compound.
APPEARS IN
संबंधित प्रश्न
Ethane, with the molecular formula C2H6 has ______.
Give appropriate scientific reasons for Carbon tetrachloride does not conduct electricity.
Fill in the following blank with suitable word:
The form of carbon which is known as black lead is ...........
Name one covalent compound containing chlorine.
Describe the structure of diamond. Draw a simple diagram to show the arrangement of carbon atoms in diamond.
One of the following is not an allotrope of carbon. This is:
(a) diamond
(b) graphite
(c) cumene
(d) buckministerfullerene
The pencil leads are made of mainly:
(a) lithium
(b) charcoal
(c) lead
(d) graphite
A solid element X has four electrons in the outermost shell of its atom. An allotrope Y of this element is used as a dry lubricant in machinery and also in making pencil leads.
(a) What is element X?
(b) Name the allotrope Y.
(c) State whether allotrope Y is a good conductor or non-conductor of electricity.
(d) Name one use of allotrope Y (other than lubrication and pencil leads)
(e) Name two other allotropes of element X.
The following structural formula belongs to which carbon compound?
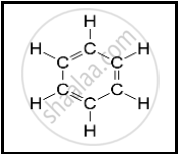
Explain the structure of Ammonium ion.
(a) Compound X consists of molecules.
Choose the letter corresponding to the correct answer from the choices (a), (b), (c) and (d) given below
X is likely to have a :
Acids dissolve in water to produce positively charged ions. Draw the structure of these positive ions.
What are Allotropes? Name any two allotropic forms of carbon. Give one use of it.
Answer the following question.
What is methane? Draw its electron dot structure. Name the type of bonds formed in this compound. Why are such compounds:
(i) poor conductors of electricity? and
(ii) have low melting and boiling points? What happens when this compound burns in oxygen?
Complete the following activity.
Write the names of the hydrocarbons for the following structural formula.
(isobutylene, cyclohexane, propene, cyclohexene, cyclopentane, benzene, propyne, isobutane, propane)
| \[\begin{array}{cc} \phantom{.........}\ce{H}\\ \phantom{.........}|\\ \ce{H - C ≡ C - C - H}\\ \phantom{.........}|\\ \phantom{.........}\ce{H} \end{array}\] |
Complete the following activity.
Write the names of the hydrocarbons for the following structural formula.
(isobutylene, cyclohexane, propene, cyclohexene, cyclopentane, benzene, propyne, isobutane, propane)
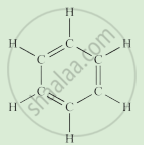 |
Which of the following compounds of carbon does not consist of ions?
Which of the following compound(s) possesses a high melting point?
The electron dot structure of chlorine molecule is:
Explain dipole (polar) molecule by taking hydrogen chloride as an example.
