Advertisements
Advertisements
Question
Give one example, state what are covalent compounds?
Solution
Covalent compounds are compounds that are formed by the sharing of electrons between two or more atoms.
Example: Hydrogen (H2) is a covalent compound, where each hydrogen atom shares one electron to form a stable compound.
APPEARS IN
RELATED QUESTIONS
What are covalent compounds?
Give reason why carbon compounds are generally poor conductors of electricity.
State the type of bonding in the following molecule.
Water
State whether the following statement is true or false:
Diamond and graphite are the covalent compounds of carbon element (C)
Fill in the blank in the following sentence:
The number of single covalent bonds in C2H2 molecule are ...........
Fill in the following blank with suitable word:
The general formula CnH2n for cycloalkanes is the same as that of ...........
Give two general properties of ionic compounds and two those of covalent compounds.
Give the formulae of the chlorides of the elements X and Y having atomic numbers of 3 and 6 respectively. Will the properties of the two chlorides be similar or different? Explain your answer.
State the type of bonding in the following molecule.
Water
Draw electron - dot structure and structural formula of methane.
Explain the following:
Non-polar covalent compounds are insoluble in water.
Compare the compounds carbon tetrachloride and sodium chloride with regard to solubility in water and electrical conductivity.
Acids dissolve in water and produce positively charged ions. Draw the structure of these positive ions.
Explain why carbon tetrachloride does not dissolve in water.
State the type of bond formed when the combining atom has small E.N. difference.
Explain the following:
Water is a polar covalent molecule.
The molecular masses of a carbon compound spread over a range of _______.
Write a short note.
Characteristics of carbon
Complete the following activity.
Write the names of the hydrocarbons for the following structural formula.
(isobutylene, cyclohexane, propene, cyclohexene, cyclopentane, benzene, propyne, isobutane, propane)
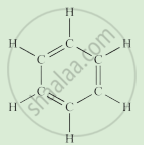 |
Complete the following activity.
Write the names of the hydrocarbons for the following structural formula.
(isobutylene, cyclohexane, propene, cyclohexene, cyclopentane, benzene, propyne, isobutane, propane)
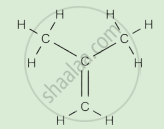 |
