Advertisements
Advertisements
प्रश्न
Give the structure of the product you would expect when the following alcohol reacts with SOCl2.
2-Methylbutan-2-ol
उत्तर
\[\begin{array}{cc}
\ce{CH3}\phantom{............................}\ce{CH3}\phantom{................}\\
|\phantom{................................}|\phantom{..................}\\
\ce{CH3 - C - CH2CH3 + SOCl2 ->[\Delta]CH3 - C - CH2CH3 + SO2 + HCl}\\
|\phantom{................................}|\phantom{..................}\\
\ce{OH}\phantom{......................}\ce{\underset{2-chloro-2-methylbutane}{Cl}}\phantom{.........}\
\end{array}\]
संबंधित प्रश्न
Explain how does the −OH group attached to a carbon of benzene ring activate it towards electrophilic substitution?
Acidity of phenol is due to ____________.
The ionization constant of phenol is higher than that of ethanol because ____________.
What is the correct order of reactivity of alcohols in the following reaction?
\[\ce{R-OH + HCl ->[ZnCl2] R-Cl + H2O}\]
Which of the following statements is true:
Out of o-nitrophenol and o-cresol which is more acidic?
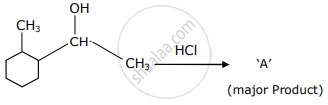
Which is the final product ‘A’ (major) in the given reaction?
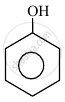
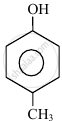
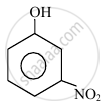
In the following compounds:
 |
 |
 |
 |
| (I) | (II) | (III) | (IV) |
The order to acidity is ______.
For the pair phenol and cyclohexanol, answer the following:
Why is phenol more acidic than cyclohexanol?
Give the structure of the product you would expect when the following alcohol reacts with HCl–ZnCl2.
2-Methylbutan-2-ol
