Advertisements
Advertisements
प्रश्न
How is acetic acid prepared from acetylene?
उत्तर
Acetylene is first converted to acetaldehyde by passing through 40% H2SO4 at 60°C in the presence of 1% HgSO4.
\[\ce{C2H2 + H2O ->[H2SO4(dil)][HgSO4] CH3CHO}\]
The acetaldehyde is then oxidised to acetic acid in the presence of catalyst manganous acetate at 70°C.
\[\ce{\underset{Acetaldehyde}{CH3CHO} + O2 ->[\Delta][Catalyst] \underset{Acetic acid}{2CH3COOH}}\]
APPEARS IN
संबंधित प्रश्न
Complete the following chemical equations :CH3COOH + Na2CO3 →
Give a reason for Conductivity of dilute hydrochloric acid is greater than that of acetic acid
Fill in the following blank with suitable word:
The next higher homologue of ethanol is ...............
Describe a test for carboxylic acid.
What is an oxidising agent?
Name the product formed and give an appropriate chemical equation for the following:
Ethanol oxidised by acidified potassium dichromate?
How Will You Carry Out the Following Conversions?
Ehane to acitic acid
Write balanced euation for the following :
Ethane is burnt in air.
Which of these is not an organic acid?
Observe the diagram given below and answer the questions:
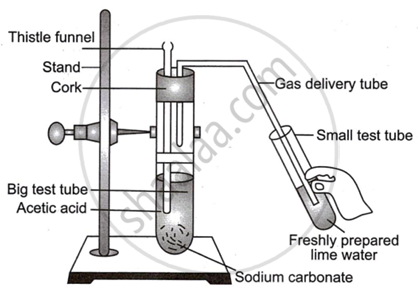
- Name the reactants in this reaction.
- Which gas comes out as effervescence in the bigger test tube?
- What is the colour change in the lime water?
- In the above experiment instead of sodium carbonate which chemical can be used to get same products?
- Write the use of acetic acid.
