Advertisements
Advertisements
प्रश्न
How will you identify?
Chlorine gas
उत्तर
It is a greenish yellow coloured gas with sharp pungent smell. It turns moist blue litmus paper red and finally bleaches it.
APPEARS IN
संबंधित प्रश्न
Complete and balance the following reaction, state whether dilute or conc. acid is used.
\[\ce{Pb(NO3)2 + HCl ->}\]
Name the following: A greenish yellow gas.
Give a balanced equation when dilute hydrochloric acid is added to : Zinc Metal
Name the gas evolved when dilute hydrochloric acid is added to: Lead (II) sulphide
Name the gas evolved when dilute hydrochloric acid is added to: Potassium bisulphite
Give a balanced equation when dilute hydrochloric acid is added to : Magnesium bicarbonate
Convert Hydrochloric acid to nascent chlorine.
Complete and balance the following reaction, state whether dilute or conc. acid is used.
NH4OH + HCl ⟶
Complete and balance the following reaction, state whether dilute or cone. acid is used.
\[\ce{NH4OH + HCl->}\]
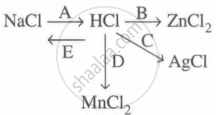
Study the flow chart and give balanced equations with conditions for the conversions A, B, C, D and E.