Advertisements
Advertisements
प्रश्न
Complete and balance the following reaction, state whether dilute or conc. acid is used.
\[\ce{Pb(NO3)2 + HCl ->}\]
उत्तर
\[\ce{Pb(NO3)2 +2HCl -> PbCl2 +2HNO3}\]
Dilute HCl is used.
APPEARS IN
संबंधित प्रश्न
Name a black metallic oxide which reacts with hydrochloric acid to give a coloured solution.
Complete and balance the following reaction, state whether dilute or conc. acid is used.
\[\ce{Pb3O4 + HCl ->}\]
Complete the following reaction and balance them.
Zn+ 2HCl ⟶
Choose the correct answer from the options given below:
Which of the following statement is not correct ?
How will you identify?
Chloride ion
Convert Hydrochloric acid to nascent chlorine.
Complete and balance the following reaction, state whether dilute or cone. acid is used.
\[\ce{NH4OH + HCl ->}\]
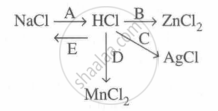
Study the flow chart and give balanced equations with conditions for the conversions A, B, C, D and E.
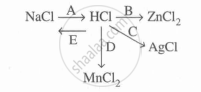
Study the flow chart and give balanced equations with conditions for the conversions A, B, C, D and E.
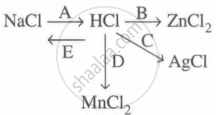
Study the flow chart and give balanced equations with conditions for the conversions A, B, C, D and E.