Advertisements
Advertisements
प्रश्न
Complete and balance the following reaction, state whether dilute or conc. acid is used.
\[\ce{NaHSO3 + HCl ->}\]
उत्तर
APPEARS IN
संबंधित प्रश्न
Name the following: A greenish yellow gas.
Name the gas evolved when dilute hydrochloric acid is added to: Lead (II) sulphide
Give a balanced equation when dilute hydrochloric acid is added to : Potassium bisulphite
How will you identify?
Chloride ion
- Give only one suitable chemical test to identify the following gases.
- Ammonia
- Sulphur dioxide
- Hydrogen chloride
- Chlorine
- Carbon dioxide
- Oxygen
- Hydrogen
- Select a basic gas mentioned in Q. (a). How is the basic nature suspected?
- Select acidic gases from the gases mentioned in Q. (a). How is the acidic nature suspected?
- The two gases A and B are bleaching agents. A is greenish-yellow and bleaches due to its oxidizing property while B is a colourless gas that bleaches due to reduction. Identify A and B?
- Which gas turn blue cobalt chloride paper light pink?
Give one similarity in the test between- Cl2 and HCl
- SO2 and CO2.
Convert Hydrochloric acid to nascent chlorine.
Study the flow chart and give balanced equations with conditions for the conversions A, B, C, D and E.
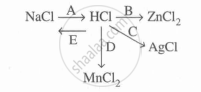
Complete and balance the following reaction; state whether it is dilute or cone. acid is used.
\[\ce{NH_4OH + HCl ->}\]
Complete and balance the following reaction, state whether dilute or conc. acid is used.
\[\ce{NH4OH + HCl ->}\]
Complete and balance the following reaction, state whether dilute or conc. acid is used.
NH4OH + HCl ⟶
