Advertisements
Advertisements
प्रश्न
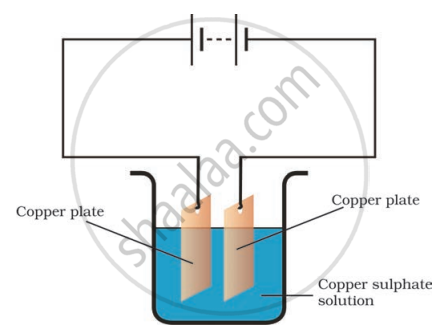
If you pass current through copper sulphate solution, copper gets deposited on the plate connected to the ______ terminal of the battery.
उत्तर
If you pass current through copper sulphate solution, copper gets deposited on the plate connected to the negative terminal of the battery.
Explanation:
When an electric current passes through a copper sulphate solution, the solution decomposes into positively charged copper ions and negatively charged sulphate ions. These positively charged copper ions are attracted to the plate connected to the negative terminal of a battery.
APPEARS IN
संबंधित प्रश्न
Prepare a list of objects around you that are electroplated.
The process that you saw in the image is used for the purification of copper. A thin plate of pure copper and a thick rod of impure copper are used as electrodes. Copper from impure rod is sought to be transferred to the thin copper plate. Which electrode should be attached to the positive terminal of the battery and why?
Electroplating is an example for ______.
Boojho and Paheli performed experiments taking similar bulbs and cells but two different solutions A and B as shown in the figure.
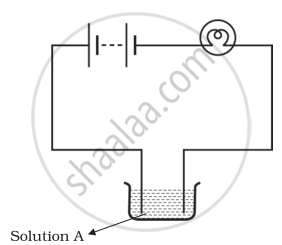 |
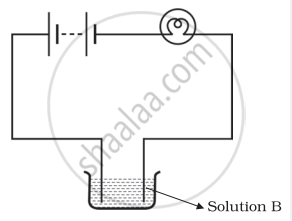 |
| Boojho's Experiment | Paheli's Experiment |
| (A) | (B) |
They found that the bulb in the set up A glows more brightly as compared to that of the set up B. You would conclude that
When electric current is passed through a conducting solution, there is a change of colour of the solution. This indicates
Which of the following metals is used in electroplating to make objects appear shining?
Paheli set up an experiment using liquid A in the beaker as shown in the figure. She observed that the bulb glows. Then, she replaced the liquid A by another liquid B. This time the bulb did not glow. Boojho suggested replacing the bulb by an LED. They observed that the LED glows. Explain.
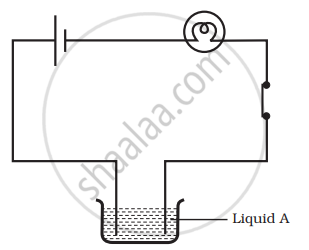
In the circuit given in the figure,
Boojho observed that copper is deposited on the electrode connected to the negative terminal of the battery.
Paheli tried to repeat the same experiment. But she could find only one copper plate. Therefore, she took a carbon rod as negative electrode. Will copper be still deposited on the carbon rod? Explain your answer.
A chemical reaction happens when electricity passes through various conducting liquids.
Match the following
| 1. | Anode | a. | Conducting solution |
| 2. | Cathode | b. | Positive terminal |
| 3. | Ions | c. | Negative terminal |
| 4. | Electrolyte | d. | Positively or negatively charged |
