Advertisements
Advertisements
प्रश्न
Illustrate Sandmeyer's reaction with an equation.
उत्तर
In benzene diazonium chloride (C6H5N2Cl) the Cl–, Br– and CN– nucleophiles can easily be introduced in the benzene ring in the presence of Cu(I) ion. This reaction is called the Sandmeyer reaction. It can be illustrated as
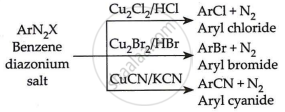
APPEARS IN
संबंधित प्रश्न
Give the structures of A, B and C in the following reactions:
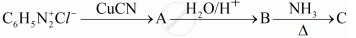
Write the structures of main products when benzene diazonium chloride `(C_6H_5N_2^(+)Cl^-)`reacts with the following reagents:
CuCN/KCN
Write the structures of main products when benzene diazonium chloride `(C_6H_5N_2^(+)Cl^-)`reacts with the following reagents:
H2O
Write the structures of main products when benzene diazonium chloride `(C_6H_5N_2^(+)Cl^-)`reacts with the following reagents:
CH3CH2OH
Write the structures of A, B and C in the following:
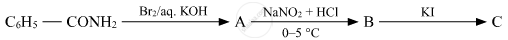
Give the structures of A, B and C in the following reaction:
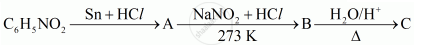
The reaction \[\ce{Ar\overset{+}{N2}Cl- ->[Cu/HCl] ArCl + N2 + CuCl}\] is anmed as ______.
Which of the following cannot be prepared by Sandmeyer’s reaction?
(i) Chlorobenzene
(ii) Bromobenzene
(iii) Iodobenzene
(iv) Fluorobenzene
Which of the following statements are correct:
(P) C6H5N=CH-C6H5 is a Schiff’s base.
(Q) A dye is obtained by the reaction of aniline and C6H5N=NCl.
(R) C6H5CH2NH2 on treatment with [NaNO2 + HCl] gives diazonium salt.
(S) p-Toluidine on treatment with [HNO2 + HCl] gives diazonium salt.
Write the structures of A, B and C in the following reaction:

