Advertisements
Advertisements
Question
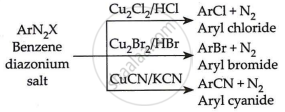
Illustrate Sandmeyer's reaction with an equation.
Solution
In benzene diazonium chloride (C6H5N2Cl) the Cl–, Br– and CN– nucleophiles can easily be introduced in the benzene ring in the presence of Cu(I) ion. This reaction is called the Sandmeyer reaction. It can be illustrated as
APPEARS IN
RELATED QUESTIONS
Give the structures of A, B and C in the following reactions:
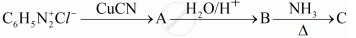
Write the structures of main products when benzene diazonium chloride `(C_6H_5N_2^(+)Cl^-)`reacts with the following reagents:
CuCN/KCN
Write the structures of main products when benzene diazonium chloride `(C_6H_5N_2^(+)Cl^-)`reacts with the following reagents:
H2O
Write the structures of main products when benzene diazonium chloride `(C_6H_5N_2^(+)Cl^-)`reacts with the following reagents:
CH3CH2OH
The reaction \[\ce{Ar\overset{+}{N2}Cl- ->[Cu/HCl] ArCl + N2 + CuCl}\] is anmed as ______.
Arrange the following compounds in increasing order of dipole moment.
\[\ce{CH3CH2CH3, CH3CH2NH2, CH3CH2OH}\]
Which of the following is not correct?
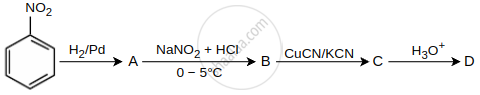
In a set of reactions, nitrobenzene gave a product D. Identify the product D.
The conversion of benzene diazonium chloride to bromobenzene can be accomplished by ______.
Write the structures of A, B and C in the following reaction:

